Question: Grignard reagents react with oxetane, a four-membered cyclic ether, to yield primary alcohols, but the reaction is much slower than the corresponding reaction with ethylene
Grignard reagents react with oxetane, a four-membered cyclic ether, to yield primary alcohols, but the reaction is much slower than the corresponding reaction with ethylene oxide. Suggest a reason for the difference in reactivity between oxetane and ethyleneoxide.
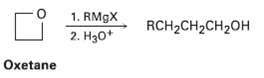
1. RM9X 2. H30* RCH2CH2CH2OH Oxetane
Step by Step Solution
3.40 Rating (172 Votes )
There are 3 Steps involved in it
The mechanism of Grignard addition to oxetane is the same as the mec... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-E (81).docx
120 KBs Word File


