Question: In sample problem 39-7 we showed that the radial probability density for the ground state of the hydrogen atom is a maximum when r =
In sample problem 39-7 we showed that the radial probability density for the ground state of the hydrogen atom is a maximum when r = a, where a is the Bohr radius, show that the average value of r, defined as has the value 1.5a. In this expression for r avg, each value of P(r) is weighted with the value of r at which it occurs. Note that the average value of r is greater than the value of r for which P(r) is amaximum.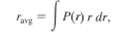
P(r) r dr. Favg
Step by Step Solution
3.56 Rating (170 Votes )
There are 3 Steps involved in it
The radial probability function for the ground state of hydrogen ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
18-P-M-P-P-M-W (139).docx
120 KBs Word File


