Question: Irreversible first-order reaction in a continuous reactor, a well-stirred reactor of volume V is initially completely filled with a solution of solute A in a
Irreversible first-order reaction in a continuous reactor, a well-stirred reactor of volume V is initially completely filled with a solution of solute A in a solvent S at concentration CAO. At time t = 0, an identical solution of a in S is introduced at a constant mass flow rate w. A small constant stream of dissolved catalyst is introduced at the same time, causing A to disappear according to an irreversible first-order reaction with rate constant k'''1 sec the rate constant may be assumed independent of composition and time. Show that the concentration of A in the reactor (assumed isothermal) at any time is in which t0 = [(w/pV) + k'''1].
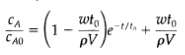
wto pV, wto -t/ta + /tot pV CA CAO
Step by Step Solution
3.29 Rating (155 Votes )
There are 3 Steps involved in it
Irreversible firstorder reaction in a continuous reactor The unsteady state ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
6-E-C-E-T-P (340).docx
120 KBs Word File


