Ninety-five percent of the acetone vapor in an 85 vol% air stream is to be absorbed by
Question:
Ninety-five percent of the acetone vapor in an 85 vol% air stream is to be absorbed by countercurrent contact with pure water in a valve-tray column with an expected overall tray efficiency of 50%. The column will operate essentially at 20?C and 101 kPa pressure. Equilibrium data for acetone-water at these conditions are:Mole percent acetone in water 3.30 7.20 11.7 17.1Acetone partial pressure in air, torr 30.00 62.80 85.4 103.0Calculate:(a) The minimum value of L'/ V', the ratio of moles of water per mole of air.(b) The number of equilibrium stages required using a value of L'/ V' of 1.25 times the minimum.(c) The concentration of acetone in the exit water. From Table 5.2 for N connected equilibrium stages, there are 2N + 2C + 5 degrees of freedom. Specified in this problem areThe remaining specification is the feed flow rate, which can be taken on a basis of 100kmol/h.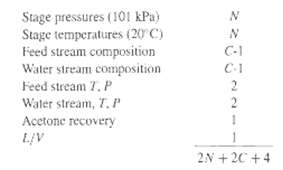
Step by Step Answer:






