Question: Polymer scientists often report their data in rather strange units. For example, in the determination of molar masses of polymers in solution by osmometry, osmotic
Polymer scientists often report their data in rather strange units. For example, in the determination of molar masses of polymers in solution by osmometry, osmotic pressures are often reported in grams per square centimeter (g cm") and concentrations in grams per cubic centimeter (g cm3).
(a) With these choices of units, what would be the units of R in the van't Hoff equation?
(b) The data in the table below on the concentration dependence of the osmotic pressure of polyisobutene in chlorobenzene at 25°C have been adapted from J. Leonard and H. Daoust (J. Polymer Sci. 57, 53 (1962». From these data, determine the molar mass of polyisobutene by plotting Tllc against c.
(c) Theta solvents are solvents for which the second osmotic coefficient is zero; for 'poor' solvents the plot is linear and for good solvents the plot is nonlinear. From your plot, how would you classify chlorobenzene as a solvent for polyisobutene? Rationalize the result in terms of the molecular structure of the polymer and solvent.
(d) Determine the second and third osmotic virial coefficients by fitting the curve to the virial form of the osmotic pressure equation.
(e) Experimentally, it is often found that the virial expansion can be represented as II/c =RT/M (1 +B'c +gB'2c'2+...) and in good solvents, the parameter g is often about 0.25. With terms beyond the second power ignored, obtain an equation for (ITIe) 1/2 and plot this quantity against c. Determine the second and third virial coefficients from the plot and compare to the values from the first plot. Does this plot confirm the assumed value of g?
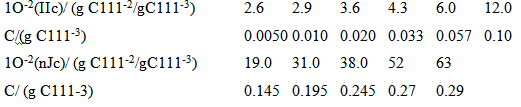
10-(Ilc) (g C111/@C111*) 2.6 2.9 3.6 4.3 6.0 12.0 C(g C1113) 10-(nJc)/ (g C111-2/gC111-3) 0.0050 0.010 0.020 0.033 0.057 0.10 63 38.0 52 19.0 31.0 0.145 0.195 0.245 0.27 C/ (g C111-3) 0.29
Step by Step Solution
3.48 Rating (168 Votes )
There are 3 Steps involved in it
By the vant Hoff equation 540 CRT M II BRT vA vA 400 300 200 100 3 N 00 00 8 2 02 04 06 Adm mol 4 6 Adm mol 8 08 10 Division by the standard accelerat... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
57-C-PC-E (147).docx
120 KBs Word File


