Question: So-called stabilizers are distillation columns that are often used in the petroleum industry to perform relatively easy separations between light components and considerably heavier components
So-called stabilizers are distillation columns that are often used in the petroleum industry to perform relatively easy separations between light components and considerably heavier components when one or two single-stage flashes are inadequate. An example of a stabilizer is shown in figure for the separation of H2, methane, and ethane from benzene, toluene, and xylenes. Such columns can be difficult to calculate because a purity specification for the vapor distillate cannot be readily determined. Instead, it is more likely that the designer will be told to provide a column with 20 to 30 trays and a water-cooled partial condenser to provide 100?F reflux at a rate that will provide sufficient boilup at the bottom of the column to meet the purity specification there. It is desired to more accurately design the stabilizer column. The number of theoretical stages shown are just a first approximation and may be varied. Strive to achieve a bottoms product with no more than 0.05 mol% methane plus ethane and a vapor distillate temperature of about 100?F. These specifications may be achieved by varying the distillate rate and the reflux ratio. Reasonable initial estimates for these two quantities are 49.4 lbmol/h and 2. Assume a tray efficiency of 70%.
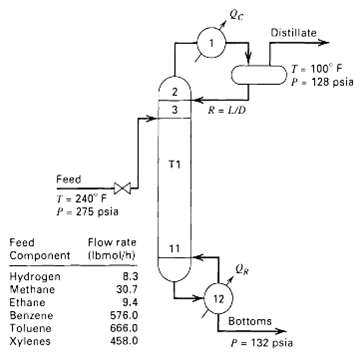
Distillate T- 100 F P. 128 psia R= LID T1 Feed T- 240" F P- 275 psia Feed Flow rate 11 Component (Ibmol/h) Hydrogen Methane 8.3 30.7 12 Ethane Benzene Toluene Xylenes 9.4 576.0 666.0 458.0 Bottoms P = 132 psia
Step by Step Solution
3.55 Rating (172 Votes )
There are 3 Steps involved in it
A valve is used to drop the feed pressure to column pressure The calculations were made with the Tower model Insideout method of Chemcad It was unders... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (397).docx
120 KBs Word File


