Prove the general thermodynamic relationship [left(frac{partial c_{p}}{partial p} ight)_{T}=-Tleft(frac{partial^{2} v}{partial T^{2}} ight)_{p}] and evaluate an expression for
Question:
Prove the general thermodynamic relationship
\[\left(\frac{\partial c_{p}}{\partial p}\right)_{T}=-T\left(\frac{\partial^{2} v}{\partial T^{2}}\right)_{p}\]
and evaluate an expression for the variation of \(c_{p}\) with pressure for a van der Waals gas which obeys a law
\[p=\frac{\Re T}{v_{m}-\mathrm{b}}-\frac{\mathrm{a}}{v_{m}^{2}}\]
where the suffix, \(m\), indicates molar quantities and \(\Re\) is the Universal gas constant.
It is possible to represent the properties of water by such an equation, where
\[\mathrm{a}=5.525 \frac{\left(\mathrm{m}^{3}\right)^{2} \mathrm{bar}}{(\mathrm{kmol})^{2}} ; \quad \mathrm{b}=0.03042 \frac{\mathrm{m}^{3}}{\mathrm{kmol}} ; \Re=8.3143 \mathrm{~kJ} / \mathrm{kmol} \mathrm{K} .\]
Evaluate the change in \(c_{p}\) as the pressure of superheated water vapour is increased from 175 bar to 200 bar at a constant temperature of \(425^{\circ} \mathrm{C}\). Compare this with the value you would expect from the steam tables abstracted below.
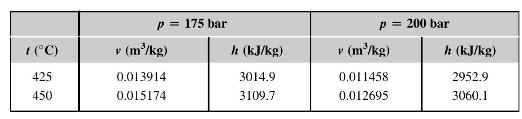
What is the value of \(\left(\frac{\partial c_{p}}{\partial p}\right)_{T}\) of a gas which obeys the Clausius equation of state, \(p=\frac{\Re T}{v_{m}-\mathrm{b}}\) ?
Step by Step Answer:

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan





