As noted in Section 13.5, the results provided for the toluene hydrodealkylation process are based on the
Question:
As noted in Section 13.5, the results provided for the toluene hydrodealkylation process are based on the SRK model for both enthalpy and phase equilibria. Simulate the benzene column (T-101) with the PR model instead, using the shortcut simulation module and the specifications given in Table 13.2 and the conditions of feed stream (10) given in Example 13.2. Determine the BIPs for the PR model used by the simulator. Rerun the simulation with all the BIPs set to zero. Compare the results.
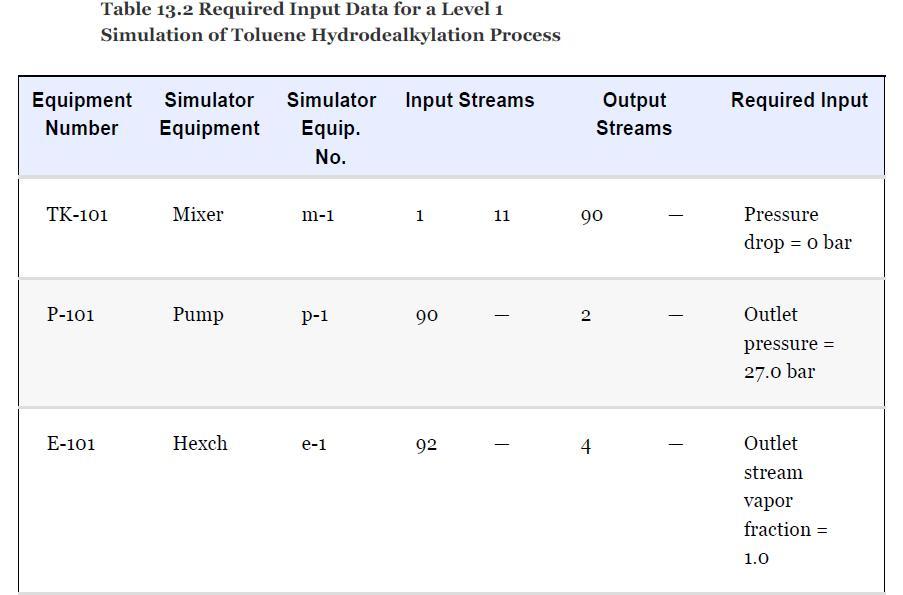
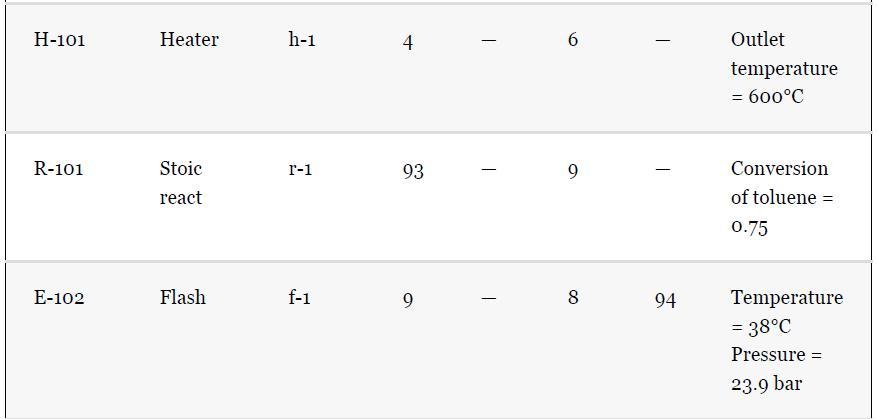
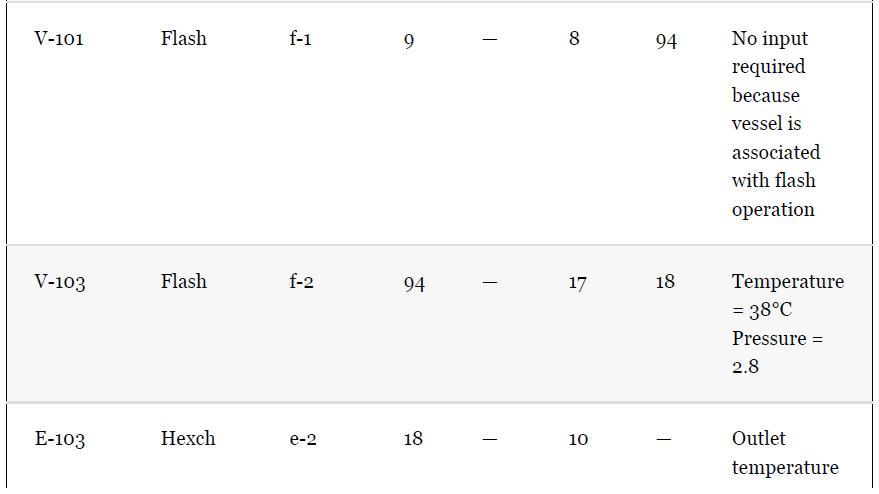
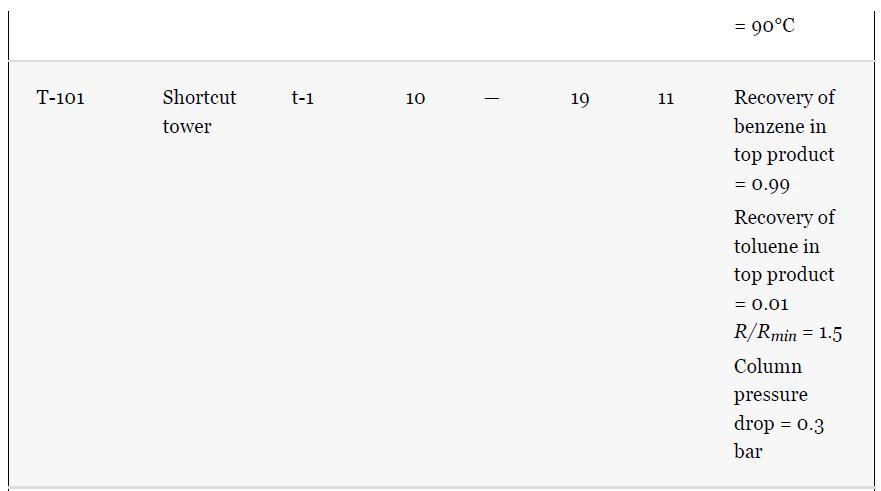
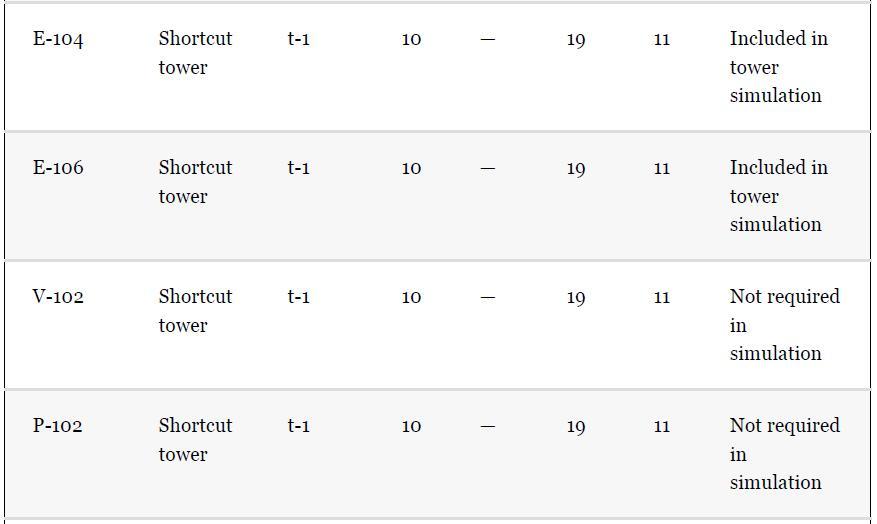
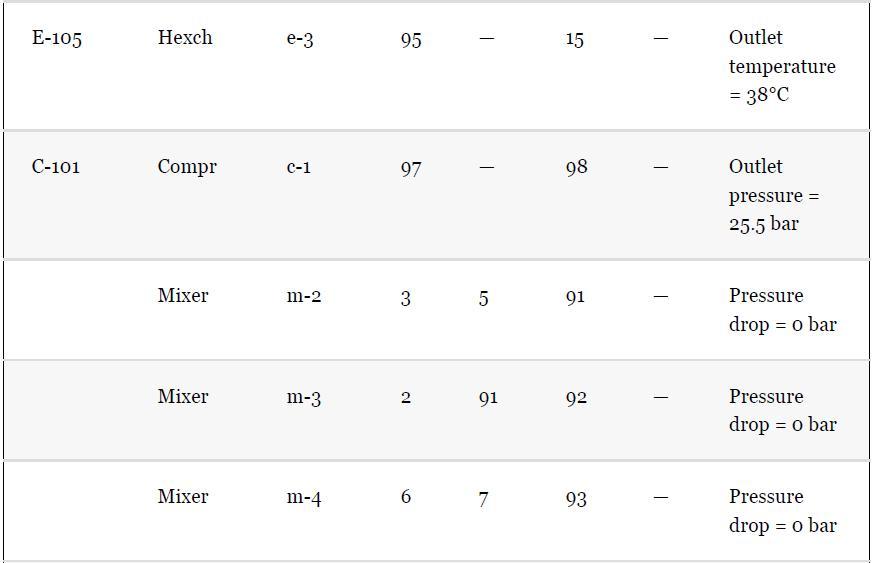
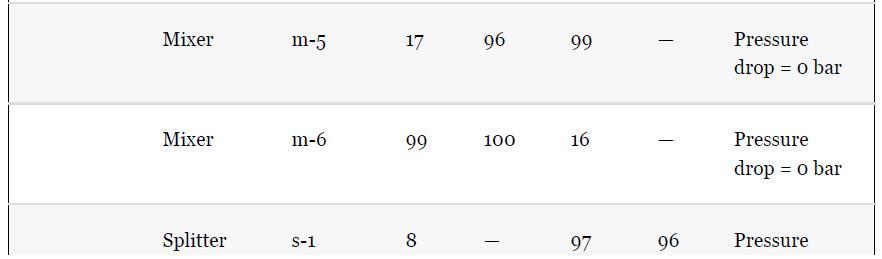
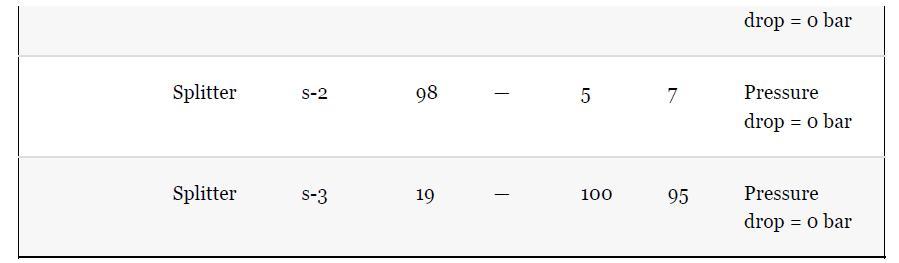
Example 13.2
Even though the ions do not directly participate in the VLE of an electrolyte system, why does their presence affect the VLE of an electrolyte system?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





