Consider Example 17.4, in which methanol is produced in a PFR. After being cooled, the reactor effluent
Question:
Consider Example 17.4, in which methanol is produced in a PFR. After being cooled, the reactor effluent is now sent to a flash vessel, V-1103, as shown in Figure P17.9. The overhead gas stream from V-1103 is sent to a compressor where it is compressed to 15 bar. The bottom liquid is pumped by P- 1101. The control valve, CV-1105, is used for maintaining the level of V-1103. The material of construction is carbon steel for all equipment. Assume the compressor to be isentropic with 80% efficiency. The efficiency of P-1101 is also 80%.
Figure P17.9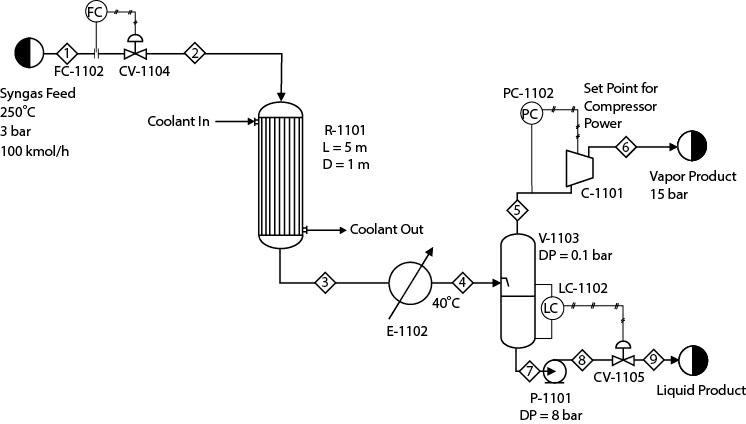
1. Develop a steady-state model of the process.
2. Develop a dynamic model. Size the required equipment following the heuristics provided in Chapter 11. Consider the metal mass of V-1103. Heat loss to the environment can be assumed to be negligible. Tune the controllers FC-1102, LC-1102, and PC-1102 one at a time. Now step decrease the syngas flow to 60 kmol/h. As you have tuned the controllers one at a time, does it perform satisfactorily when the controllers interact with each other? If not, what changes should you make to achieve satisfactory performance in case of loop interactions?
3. Now double the proportional gain of PC-1102. What happens to the performance of LC-1102? Why? Retune LC-1102 to achieve satisfactory performance.
Example 17.4
Synthesis gas composed of CO, CO2, H2, and H2O is sent to a plug-flow reactor, R-1101, through a feed valve for producing methanol as shown in Figure E17.4(a). The following reactions take place [10, 11]: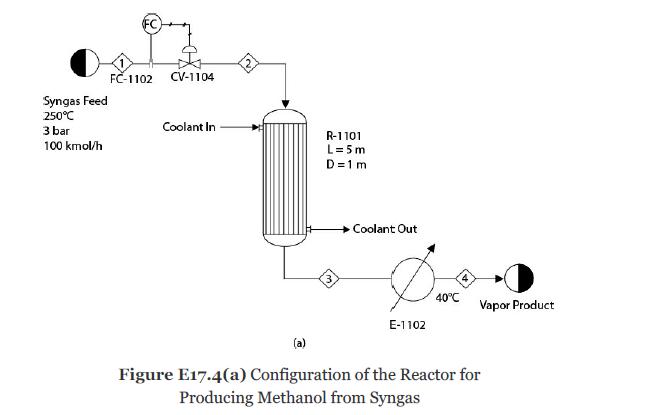
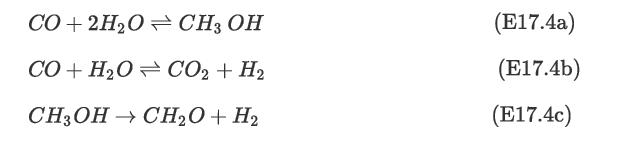
The corresponding rate laws for these reactions are [10, 11]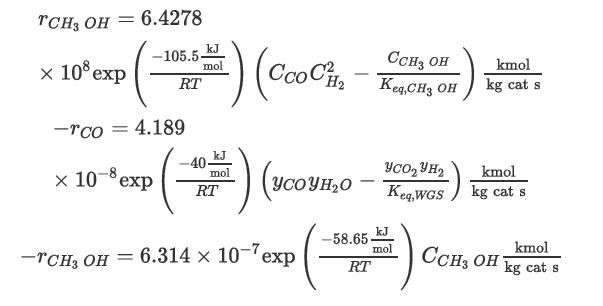
where Ci is the concentration of the species i in kmol/m3.
A coolant with a constant temperature of 350°C and the overall heat transfer coefficient is 80 W/m2°C. The reactor effluent is sent to E-1102 where it is cooled to 40°C at steady state. At steady state, the feed composition (mole fraction) and feed flowrate are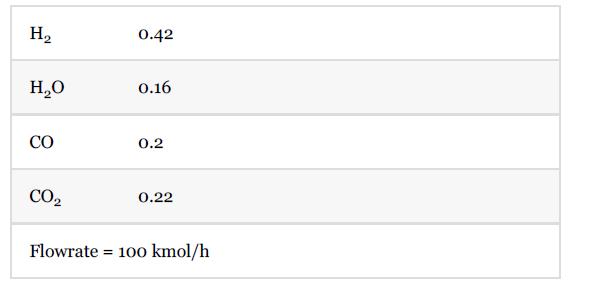
The catalyst particle density is 1800 kg/m3, and the bed voidage is 0.45. The reactor length is 5 m, and the diameter is 1 m. At steady state, the feed valve is 50% open. The feed valve is closed to 25% in a single step. Compare the dynamic responses of the methanol and H2 concentrations at the reactor outlet by using various explicit methods (such as the explicit Euler and Runge- Kutta methods) and implicit methods (such as the implicit Euler and Gear’s method of various orders).
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





