The apparatus shown in the diagram below was used to investigate the gases produced when a concentrated
Question:
The apparatus shown in the diagram below was used to investigate the gases produced when a concentrated solution of potassium chloride was electrolysed.
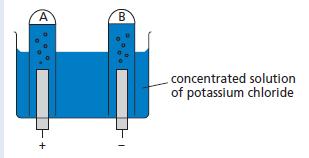
a. Name a non-metal suitable for use as electrodes.
b. Name the gas collected in A and the gas collected in B.
c. Describe how you would test the gases collected.
d. The volume of gas collected in B was slightly less than that collected in A. The teacher said the volumes should have been equal but gave a simple explanation of the ‘missing’ gas in B. What was the explanation? (Assume that the apparatus was working perfectly).
e. Write down the equations which describe the production of the gases at the electrodes in A and B.
f. (i) If the concentrated solution of potassium chloride was now replaced by dilute sodium hydroxide what gases would be produced at A and B?
(ii) In what ratio would you expect these gases to be produced?
Step by Step Answer:






