An acidified solution of hydrogen peroxide reacts with iodide ions. H 2 O 2 (aq) + 2H
Question:
An acidified solution of hydrogen peroxide reacts with iodide ions.
H2O2(aq) + 2H+(aq) + 2I–(aq) → 2H2O(l) + I2(aq)
The rate equation for this reaction is rate = [H2O2] [I–] The mechanism below has been proposed for this reaction.
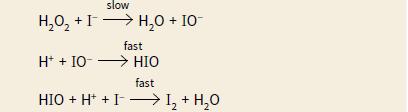
Explain why this mechanism is consistent with the rate equation.
slow H,0, +I> H,0 + IO H,0 + 10- fast H* + IO- > HIO fast HIO + H* + I -I, + H,0
Step by Step Answer:
The rate equation rate H2O2 I suggests that the reaction rate depends on both hydrogen peroxide and ...View the full answer



Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Related Video
Hydrogen peroxide can be used as a mild antiseptic to curb superficial skin infections such as athlete’s foot, but only in diluted quantities. To combat stinky feet, try soaking your feet in a solution of 1 part 3% hydrogen peroxide and 3 parts warm water for 15-20 minutes, then drying them thoroughly. This will kill odor-causing bacteria and soften your feet. To treat athlete\'s foot, you can use a similar solution, but only in diluted quantities, and soak your feet for 30 minutes. Hydrogen peroxide can also be used to keep vegetables fresh by adding 1/4 cup to a bowl of cold water, soaking the vegetables for 20-30 minutes, then draining, drying, and refrigerating them. Alternatively, you can spray vegetables with a solution of 3% hydrogen peroxide and let them stand for a few minutes before rinsing and drying. To keep leftover salad fresh, spray it with a solution of 1/2 cup water and 1 Tbsp. 3% hydrogen peroxide, drain, cover, and refrigerate.
Students also viewed these Sciences questions
-
The rate equation for the reaction between iodine and propanone is: rate = k[CH 3 COCH 3 ] [H + ] [I 2 ] 0 a. State the order of reaction with respect to iodine. b. State the overall order of...
-
Aldolase catalyzes the reaction ÎG°² for this reaction is 22.8 kJmol-1. In the cell at 37°C, the ÎG for this reaction is -5.9 kJmol-1. What is the ratio [GAP][DHAP]/[FBP]?...
-
When you calculated A in the rate equation for the reaction of kMnO 4 solution and H 2 C 2 O 4 solution. you assumed k had the same value under the conditions of determinations 1, 2, and 3. (a) What...
-
The CFO of the Jordan Microscope Corporation intentionally misclassified a downstream transportation expense in the amount of $575,000 as a product cost in an accounting period when the company made...
-
Consider population data with = 20 and = 2. (a) Compute the coefficient of variation. (b) Compute an 88.9% Chebyshev interval around the population mean.
-
The accounting staff of Best Company has assembled the following information for the year ended December 31, 2011: Cash sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
-
Youre considering an investment in Croatia that you expect will produce a 9 percent return next year, and you expect that your real rate of return on this investment will be 7 percent. What do you...
-
Stacy McGuire recently opened her own basketweaving studio. She sells finished baskets in addition to the raw materials needed by customers to weave baskets of their own. Stacy has put together a...
-
1 What is HRM 2 What is Training and Developmnt 3 What is Recruitment 4 What is Selection 5 What is HRP 6 What is Job Analysis 7 What is a Career Planning and Development 8 What is Job-Description 9...
-
1: Using variable elimination (by hand!), compute the probability that a student who did well on the test actually understood the material, that is, compute P(+u| + e). 2: For the above Bayesian...
-
a. State which pairs of substances i to iv below might catalyse the reaction: S 2 O 8 2 (aq) + 2I (aq) 2SO 4 2 (aq) + I 2 (aq) Explain your answer. i. Ni 2+ (aq) / Ni(s) E = 0.25 V ii. Mn 3+ (aq)...
-
a. Write the rate equation for the acid-catalysed reaction of iodine with propanone. b. Use your rate equation and the information in Table 22.9 (experiment 1) to calculate a value for the rate...
-
On January 1, 2017, Alan King decided to transfer an amount from his chequing account into a savings account that later will provide $80,000 to send his son to university (four years from now). The...
-
1 Express the extended fraction at the right as a simple 1 2+ fraction in lowest terms. 1 2+ 2+- 2
-
A company has the following capital structure: 1. Target weightings: 30% debt, 20% preferred stock,50%common equity 2. Tax rate 30% 3. The cost of debt 6.5% 4. Cost of preferred stock is 8% 5. The...
-
A commonly prescribed drug on the market for relieving nervous tension is believed to be only 60% effective. What is the probability that in a random sample of 100 adults, more than 70% (more than 70...
-
Find the sum of the terms in the arithmetic sequence below, Sn: 2, -2, -6, -10,..., -30 S = Sn (Simplify your answer.)
-
What factors should you consider when coming up with an OU design?
-
A steep cliff west of Lydia's home reflects a 1020-kHz radio signal from a station that is 74 km due east of her home. If there is destructive interference, what is the minimum distance of the cliff...
-
You work as an operations consultant for a textile company. Your client has a well-established distribution system in the US market. The company has hundreds of stores and four distribution centers....
-
For each of the compounds below, locate the pattern we just learned (lone pair next to a Ï bond) and draw the appropriate resonance structure: a. b. c. d. e. f. g. h. NH2
-
Draw the resonance structure(s) for each of the compounds below: a. b. c. d.
-
The formalism of the Youngs modulus is sometimes used to calculate the reversible work involved in extending or compressing an elastic material. Assume a force F is applied to an elastic rod of...
-
5. Assume that the economy of Meekland is in a long-run equilibrium with a balanced government budget. (a) Using a correctly labeled graph of aggregate supply and aggregate demand, show each of the...
-
= Choose values for Rc and RE so that the bias current through RE is approximately 2.1 mA and both transistor's VCE is approximately 4.9V (for quiescent analysis set V = V = 0V). Use Vcc=-VEE 15V,...
-
Overhead Applied to Jobs, Departmental Overhead Rates Xania Inc. uses a normal job-order costing system. Currently, a plantwide overhead rate based on machine hours is used. Xania's plant manager has...

Study smarter with the SolutionInn App


