Propanone is a liquid. It has the structure The equation for the complete combustion of propanone is:
Question:
Propanone is a liquid. It has the structure
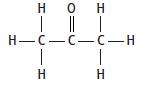
The equation for the complete combustion of propanone is:
CH3COCH3(l) + 4O2(g) → 3CO2(g) + 3H2O(l)
a. Use the following bond energies (in kJ mol–1) to calculate a value for the standard enthalpy change of this reaction:
E(C — C) = +347
E(C — H) = +413
E(O — O) = +496
E(C — O) = +805
E(O — H) = +465
b. Suggest why it would be more accurate to use bond energies that are not average bond energies in this calculation.
c. The standard enthalpy change of combustion of propanone is –1816.5 kJ mol–1. Suggest why this value differs from the value obtained using bond energies.
d. The standard enthalpy change of formation of propanone is –248 kJ mol–1.
i. Define the term standard enthalpy change of formation.
ii. Write the equation that describes the standard enthalpy change of formation of propanone.
iii. Explain why the enthalpy change of formation of propanone cannot be found by a single experiment.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris




