State the direction of the electron flow in the electrochemical cells represented by the following pairs of
Question:
State the direction of the electron flow in the electrochemical cells represented by the following pairs of half-equations. Use the data in Appendix 2 to help you.
a. F2 + 2e– ⇌ 2F– and Mn2+ + 2e– ⇌ Mn
b. Sn4+ + 2e– ⇌ Sn2+ and I2 + 2e– ⇌ 2I–
c. Cr2O72– + 14H+ + 6e– ⇌ 2Cr3+ + 7H2O and Cu2+ + 2e– ⇌ Cu
d. Ni2+ + 2e– ⇌ Ni and Fe3+ + 3e– ⇌ Fe
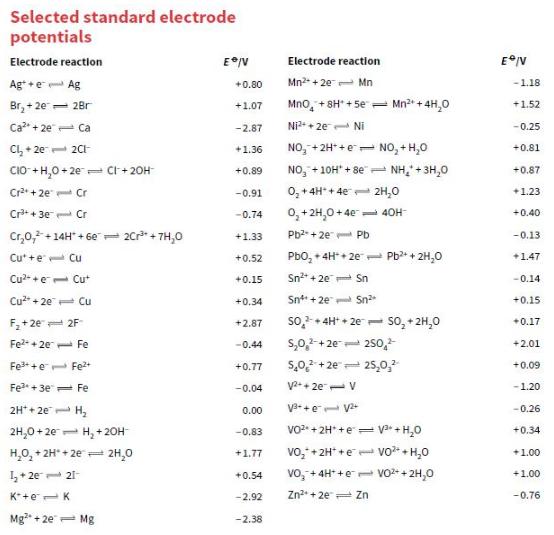
Transcribed Image Text:
Selected standard electrode potentials Electrode reaction Electrode reaction Ag+ +e- Ag Mn2 + 2e- Mn +0.80 -1.18 Br, + 2e= 28r Mno, + 8H* + 5e = Mn2* + 4H,0 +1.07 +1.52 Ca + 2e Ca -2.87 Ni2+ + 2e- Ni - 0.25 NO, +2H* +e= NO, + H,0 +0.81 Cl, + 2e 2CH +1.36 CIO +H,0+ 2e- Cr + 20H NO, +10H" + 8e"- NH,+ 3H,0 +0.89 +0.87 Cr* + 2e Cr -0.91 0, +4H* + 4e 2H,0 +1.23 Cr+ 30 Cr 0,+ 2H,0+ 4e- 4OH -0.74 +0.40 Cr,0,+14H" + 6e= 20r* + 7H,0 +1.33 Pb2 + 2e Pb -0.13 Cu* +e Cu +0.52 Pbo, + 4H* + 2e 1 Pb* + 2H,0 +1.47 Cu2- +e Cut +0.15 Sn2* + 20 Sn -0.14 Cu + 20 Cu +0.34 Snt + 2e Sn +0.15 so + 4H* + 2e so, + 2H,0 F,+2e= 2F +2.87 +0.17 s,0,-+2e- 250, 5,0,2 + 2e= 25,0, Fe2 + 20 Fe -0.44 +2.01 Fe++e- Fe2+ +0.77 +0.09 Fe*+3e Fe -0.04 V+ 2e- V -1.20 2H* + 2e H, V3-+e V -0.26 0.00 2H,0 + 2e- H, + 20H VO2 + 2H* +e = + H,0 -0,83 +0.34 H,0, + 2H* + 2e 2H,0 vo,+ 2H* +e vo+H,0 +1.77 +1.00 1+ 2e 21- vo, + 4H* +e vo+ + 2H,0 +1.00 +0.54 K*+e-K -2.92 Zn + 2e Zn -0.76 Mg + 2e- Mg -2.38
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
a The direction of electron flow in the electrochemical cell represented by the halfequat...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
Use the data in Appendix 2 to predict whether or not the following reactions are feasible. If a reaction does occur, write a balanced equation for it. a. Can MnO 4 ions oxidise Cl ions to Cl 2 in...
-
Use the data in Appendix 2 to answer these questions. a. Of the ions Ag + , Cr2 + and Fe 2+ , which one needs the strongest reducing agent to reduce it to metal atoms? b. Of the atoms Ag, Cr and Fe,...
-
Use data in Appendix C to calculate ÎH°, ÎS°, and ÎG° at 25°C for each of the following reactions. In each case show that ÎG° = ÎH° -...
-
Perhaps more surprising to Mr. Pitkin was a proposal by the VP of Marketing to make a major investment in market share by increasing promotional expenditures by $2.5 million during 1998-2000. Sales...
-
Let z be a random variable with a standard normal distribution. Find the indicated probability and shade the corresponding area under the standard normal curve. P( 0.82 z 0)
-
Braggs Bakery is building a new automated bakery in downtown Sandusky. Here are the activities that need to be completed to get the new bakery built and the equipment installed. a. Draw the project...
-
Draw a free-body diagram for the person in Exercise 8.4. Data from Exercises 8.4 Consider a person hanging motionless from a ring suspended from a cable, with the person's feet not touching the...
-
Coast to Coast Surfboards Inc. manufactures and sells two styles of surfboards, Atlantic Wave and Pacific Pounder. These surfboards are sold in two regions, East and West. Information about the two...
-
1. A planet is revolving around the sun in an elliptical orbit. The mass of planet is m, angular momentum of planet about sun is L, and length of semi major axis is a and eccentricity is e. Time...
-
In this lab, we will use the inverse kinematics to identify two functions to generate an animation of a two-link robot. As mentioned in the previous section, the lengths of the two links are a = 3 ft...
-
a. Draw a diagram of an electrochemical cell consisting of a Mn 2+ /Mn half-cell and a Pb 2+ /Pb half-cell. b. Use the data in Appendix 2 to calculate the cell voltage. c. Which half-cell is the...
-
Suggest a suitable reagent that can carry out each of the following oxidations or reductions. Use the data in Appendix 2 to help you. a. The reduction of Zn 2+ ions to Zn. b. The oxidation of Br ...
-
Corey Snieds operates two gas stations. He has just received the monthly bank statement at May 31 from Royal Bank, and the statement shows an ending balance of $23,440. Listed on the statement are an...
-
All research requires some level of funding and a budget should be included in the proposal (both quantitative and qualitative) even if outside funding is not being sought?
-
Compare and contrast standard error and margin of error.
-
A company has $90,000 in outstanding accounts receivable and it uses the allowance method to account for uncollectible accounts. Experience suggests that 4% of outstanding receivables are...
-
What type of force, either the applied force, frictional force, or sum of forces that really effects the acceleration of an object. Explain.
-
A project requires an initial investment of $50,000. The project will generate net cash flows of $15,000 at the end of the first year, $40,000 at the end of the second year, and $10,000 at the end of...
-
A hydrogen atom in its ground state is immersed in a continuous spectrum of ultraviolet light with wavelengths ranging from 96 nm to 110 nm. After absorbing a photon, the atom emits one or more...
-
You are thinking of investing in one of two companies. In one annual report, the auditors opinion states that the financial statements were prepared in accordance with generally accepted accounting...
-
Show what reagents you would use to prepare each of the following ethers via an alkoxymercuration-demercuration. a. b. c. d. OEt
-
A 2.25 mole sample of carbon dioxide, for which C P,m = 37.1 J K -1 mol -1 at 298 K, is expanded reversibly and adiabatically from a volume of 4.50 L and a temperature of 298 K to a final volume of...
-
How would you use an alkoxymercuration-demercuration to prepare dicyclopentyl ether using cyclopentene as your only source of carbon?
-
x16 AVB Express as a fraction 9y17 C reduced in its lowest terms without any radicals in the denominator
-
2. (points) Write a state diagram of floating literals in swift whose grammar has been provided floating-point-literal -> decimal-literal [decimal-fraction ] [ decimal-exponent 1...
-
Human Resources managers seek to attract and retain the best workers possible. Are your skills attractive enough to secure your dream job? In some industries, the workforce is shrinking as artificial...

Study smarter with the SolutionInn App


