The table shows the atomic number and boiling points of some noble gases. a. Use ideas about
Question:
The table shows the atomic number and boiling points of some noble gases.
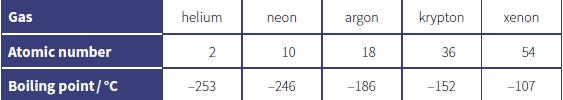
a. Use ideas about forces between atoms to explain this trend in boiling points.
b. Xenon forms a number of covalently bonded compounds with fluorine.
i. What do you understand by the term covalent bond?
ii. Draw a dot-and-cross diagram for xenon tetrafluoride, XeF4.
iii. Suggest a shape for XeF4. Explain why you chose this shape.
c. The structure of xenon trioxide is shown below.
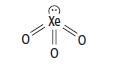
i. By referring to electron pairs, explain why xenon trioxide has this shape.
ii. Draw the structure of xenon trioxide to show the partial charges on the atoms and the direction of the dipole in the molecule.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:




