The behavior of hydrogen fluoride is unusual! For example, here are the critical properties of various hydrogen
Question:
The behavior of hydrogen fluoride is unusual! For example, here are the critical properties of various hydrogen halides:
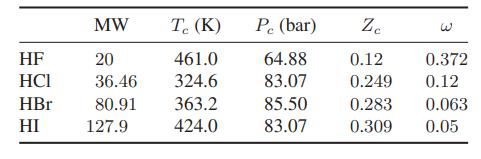
has the lowest reported critical compressibility of any species. Experimental data for the vapor pressure and the apparent molecular weight of saturated
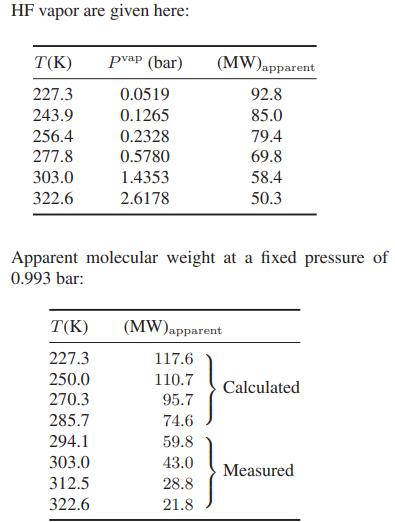
In each case the apparent molecular weight has been found by measuring the mass density of the vapor and comparing that with an ideal gas of molecular weight 20. One possible explanation for this behavior is that hydrogen fluoride associates according to the following set of reactions:
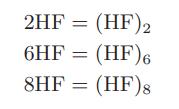
Describe how you would use these data to develop a model for HF so that you could determine the vapor-liquid equilibrium of HF and a component that did not associate.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





