Question: An extractive distillation system is separating ethanol from water using ethylene glycol as solvent. The makeup solvent is pure ethylene glycol. In Figure 8-14, ethanol
An extractive distillation system is separating ethanol from water using ethylene glycol as solvent. The makeup solvent is pure ethylene glycol. In Figure 8-14, ethanol is A product, and water is B product. The feed is 20.0 \(\mathrm{mol} \%\) ethanol, and the remainder is water with flow rate of \(100.0 \mathrm{kmol} / \mathrm{h}\). The ethanol product (column 1 distillate) is \(99.70 \mathrm{~mol} \%\) ethanol, 0.02 \(\mathrm{mol} \%\) ethylene glycol, and the remainder is water. The water product (column 2 distillate) is \(99.90 \mathrm{~mol} \%\) water, \(0.035 \mathrm{~mol} \%\) ethylene glycol, and the remainder is ethanol.
Figure 8-14
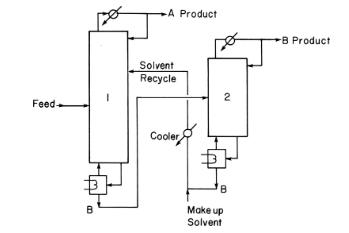
a. Find the flow rates of the makeup solvent and of the ethanol and water products.
b. Why would a separation flowsheet that uses a normal distillation column to preconcentrate the feed be chosen instead of this separation?
A Product Feed- Solvent Recycle Cooler B Make up Solvent 2 B Product
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it
Solutions Step 1 Analysis of Extractive Distillation System D16 The problem describes an extractive distillation system separating ethanol from water using ethylene glycol as the solvent We need to fi... View full answer

Get step-by-step solutions from verified subject matter experts


