Question: We are separating nitromethane and water in a distillation system consisting of two columns, two total condensers, two partial reboilers, and a liquid-liquid separator. CMO
We are separating nitromethane and water in a distillation system consisting of two columns, two total condensers, two partial reboilers, and a liquid-liquid separator. CMO is valid, and \(p=1.0 \mathrm{~atm}\). There are two feeds to the system. Feed 1 has \(F_{1}=100.0 \mathrm{kmol} / \mathrm{h}\) and \(\mathrm{z}_{1}=0.15\) water \((0.85 \mathrm{NM})\) and is a saturated liquid. Feed 2 has \(F_{2}=80.0 \mathrm{kmol} / \mathrm{h}\) and \(\mathrm{z}_{2}=0.94\) water \((0.06 \mathrm{NM})\) and is a saturated liquid.
Desired results: Water (W) product (flow rate \(\mathrm{P}_{\mathrm{W}} \mathrm{kmol} / \mathrm{h}\) ) contains \(\mathrm{x}_{\mathrm{NM}, \mathrm{PW}}=1.0 \mathrm{~mol} \% \mathrm{NM}\left(\mathrm{x}_{\mathrm{W}, \mathrm{PW}}=0.99\right)\). This column has \(abla / \mathrm{B}=0.2\). Nitromethane (NM) product (flow rate \(\mathrm{P}_{\mathrm{NM}} \mathrm{kmol} / \mathrm{h}\) ) contains \(\mathrm{x}_{\mathrm{W}, \mathrm{PNM}}=\) \(2.5 \mathrm{~mol} \% \mathrm{~W}\left(\mathrm{x}_{\mathrm{NM}, \mathrm{PNM}}=0.975\right)\). This column has \(\approx / \mathrm{B}=2.3\).
a. Find product flow rates \(\mathrm{P}_{\mathrm{NM}}\) and \(\mathrm{P}_{\mathrm{W}}\) in \(\mathrm{kmol} / \mathrm{h}\).
b. Sketch the process showing schematically where each feed is located.
c. Find the optimum feed locations and the total number of stages for both columns.
Derive your operating lines.
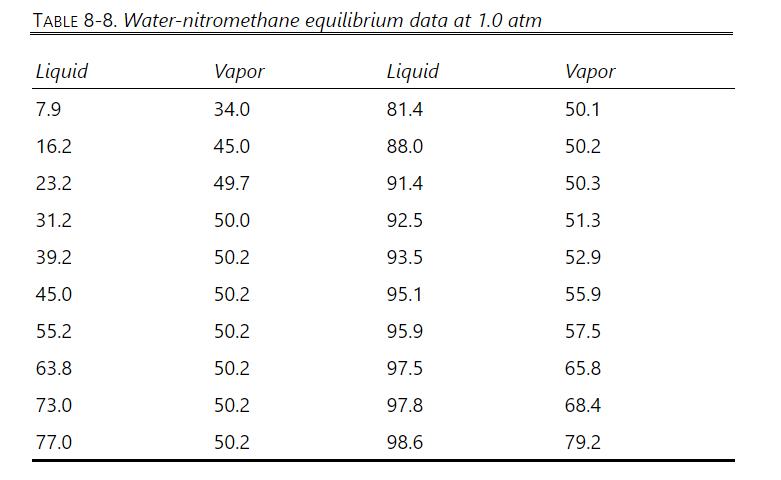
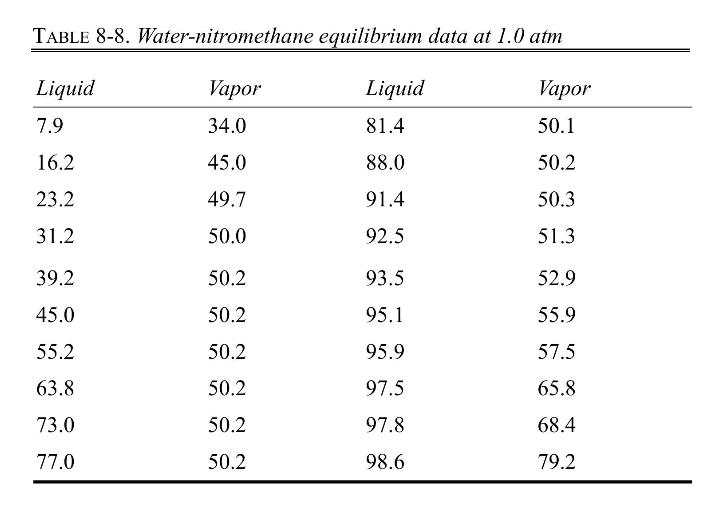
Equilibrium data are in Table 8-8, Problem 8.D16. Density data are also given in Problem 8.D16. Be sure to read the note on safety at the end of Problem 8.D16.
Problem 8.D16.
Nitromethane and water are separated in a rectifying column system with a total condenser and a liquid-liquid settler similar to Figure 8-3A, except the column is a rectifying column. The saturated vapor feed is \(7.5 \mathrm{~mol} \%\) water. The water product from the settler is \(91.4 \mathrm{~mol} \%\) water. The organic phase refluxed to the column is \(31.2 \mathrm{~mol} \%\) water. Pressure \(=1.0 \mathrm{~atm}\), feed rate \(=50.0 \mathrm{kmol} / \mathrm{h}\), and \(\mathrm{CMO}\) is valid. The desired bottoms is 2.5 \(\mathrm{mol} \%\) water.
Figure 8-3A
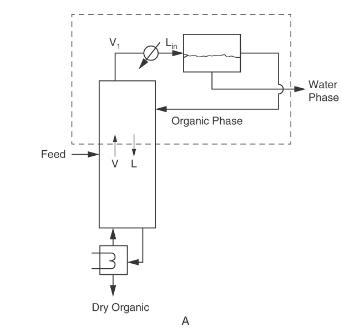 Find: \(\mathrm{x}_{\text {dist,water }}\), reflux ratio \(\mathrm{L} / \mathrm{D}, \mathrm{D}, \mathrm{B}\), and the number of equilibrium stages.
Find: \(\mathrm{x}_{\text {dist,water }}\), reflux ratio \(\mathrm{L} / \mathrm{D}, \mathrm{D}, \mathrm{B}\), and the number of equilibrium stages.
Equilibrium data are in Table 8-8. Density data are given after the table, and be sure to read the note on safety at the end of this problem.
Data: Density of nitromethane at \(25^{\circ} \mathrm{C}\) is \(1129 \mathrm{~kg} / \mathrm{m}^{3}\).
Safety note: Nitromethane (also called methyl nitrate, \(\mathrm{CH}_{3} \mathrm{NO}_{2}\) ) is dangerous. If you try to freeze the pure compound, it explodes.
TABLE 8-8. Water-nitromethane equilibrium data at 1.0 atm Liquid Vapor Liquid Vapor 7.9 34.0 81.4 50.1 16.2 45.0 88.0 50.2 23.2 49.7 91.4 50.3 31.2 50.0 92.5 51.3 39.2 50.2 93.5 52.9 45.0 50.2 95.1 55.9 55.2 50.2 95.9 57.5 63.8 50.2 97.5 65.8 73.0 50.2 97.8 68.4 77.0 50.2 98.6 79.2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


