Determine the modified Sherwood number (mathrm{Sh}_{text {gas,partial_pressure }}=frac{mathrm{k}_{mathrm{p}} mathrm{d}_{text {tube }}left(mathrm{p}_{mathrm{B}} ight)_{mathrm{lm}}}{D_{mathrm{AB}} mathrm{p}_{text {tot }}}) for gas-side-controlled
Question:
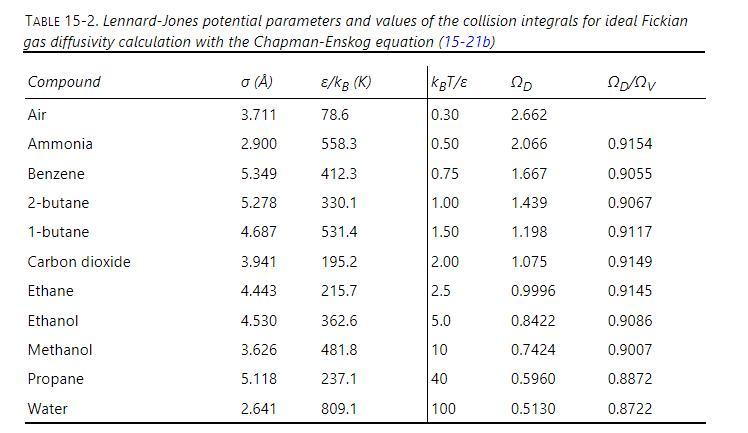
Determine the modified Sherwood number \(\mathrm{Sh}_{\text {gas,partial_pressure }}=\frac{\mathrm{k}_{\mathrm{p}} \mathrm{d}_{\text {tube }}\left(\mathrm{p}_{\mathrm{B}}\right)_{\mathrm{lm}}}{D_{\mathrm{AB}} \mathrm{p}_{\text {tot }}}\) for gas-side-controlled mass transfer for distillation of ethanol-water in a wetted wall column. Column diameter is \(10.0 \mathrm{~cm}\). Measurements are at very low ethanol concentrations where flowing liquid can be assumed to be pure water. Total pressure is 1.0 bar. Liquid water at its boiling temperature is flowing down a vertical column at a volumetric flow rate per meter of circumference of \(q=0.0000075 \mathrm{~m}^{2} / \mathrm{s}\). Ethanol is diffusing through an upward-flowing vapor (almost pure water) at 1.0 bar and its boiling temperature. The upward vapor velocity is \(0.81 \mathrm{~m} / \mathrm{s}\). Densities and viscosities of pure water liquid and pure water vapor are available in Perry's Handbook of Chemical Engineering. Use parameters in Table 15-2 to estimate diffusivity of ethanol-water in the vapor.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





