Question: Repeat Problem 15.D12 but for (mathrm{q}=0.0015 mathrm{~m}^{2} / mathrm{s}). a. Determine film thickness (delta), average vertical velocity of film, and Reynolds number. b. Determine average
Repeat Problem 15.D12 but for \(\mathrm{q}=0.0015 \mathrm{~m}^{2} / \mathrm{s}\).
a. Determine film thickness \(\delta\), average vertical velocity of film, and Reynolds number.
b. Determine average mass transfer coefficient and average Sherwood number.
c. Per meter of plate width, at what rate \((\mathrm{kg} / \mathrm{s})\) is carbon dioxide transferred into water over the length of the plate?
Problem 15.D12
Water at \(20^{\circ} \mathrm{C}\) is flowing down a \(3.0 \mathrm{~m}\) long vertical plate at a volumetric flow rate per meter of plate width of \(q=0.000005 \mathrm{~m}^{2} / \mathrm{s}\). Entering \((y=0)\) water is pure. The water is in contact with carbon dioxide gas that is saturated with water vapor at \(20^{\circ} \mathrm{C}\) (no water is evaporating). Carbon dioxide transfers into the water. At the water-gas surface \((\mathrm{z}=0)\), water and carbon dioxide are in equilibrium at the solubility limit of carbon dioxide at \(\mathrm{C}_{\mathrm{CO} 2 \text {,surf }}\). Estimate Fickian infinite dilution diffusivity by adjusting the value from Table 15-3 for the temperature difference by assuming that \(\mathrm{E}_{\mathrm{o}}=2000\) \(\mathrm{cal} / \mathrm{mol}\) in Eq. \((15-22 \mathrm{c})\). At \(20^{\circ} \mathrm{C}\), the solubility of carbon dioxide in water is \(1.7 \mathrm{~g} / \mathrm{kg}\) water.
a. Determine film thickness \(\delta\), average vertical velocity of film, and Reynolds number.
b. Is flow laminar? If no surfactant is added, do you expect ripples on the surface? If surfactant is added, do you expect ripples on the surface?
c. With no surfactant, determine average mass transfer coefficient and average Sherwood number.
d. Per meter of plate width, at what rate \((\mathrm{kg} / \mathrm{s})\) is carbon dioxide transferred into water over the length of the plate?
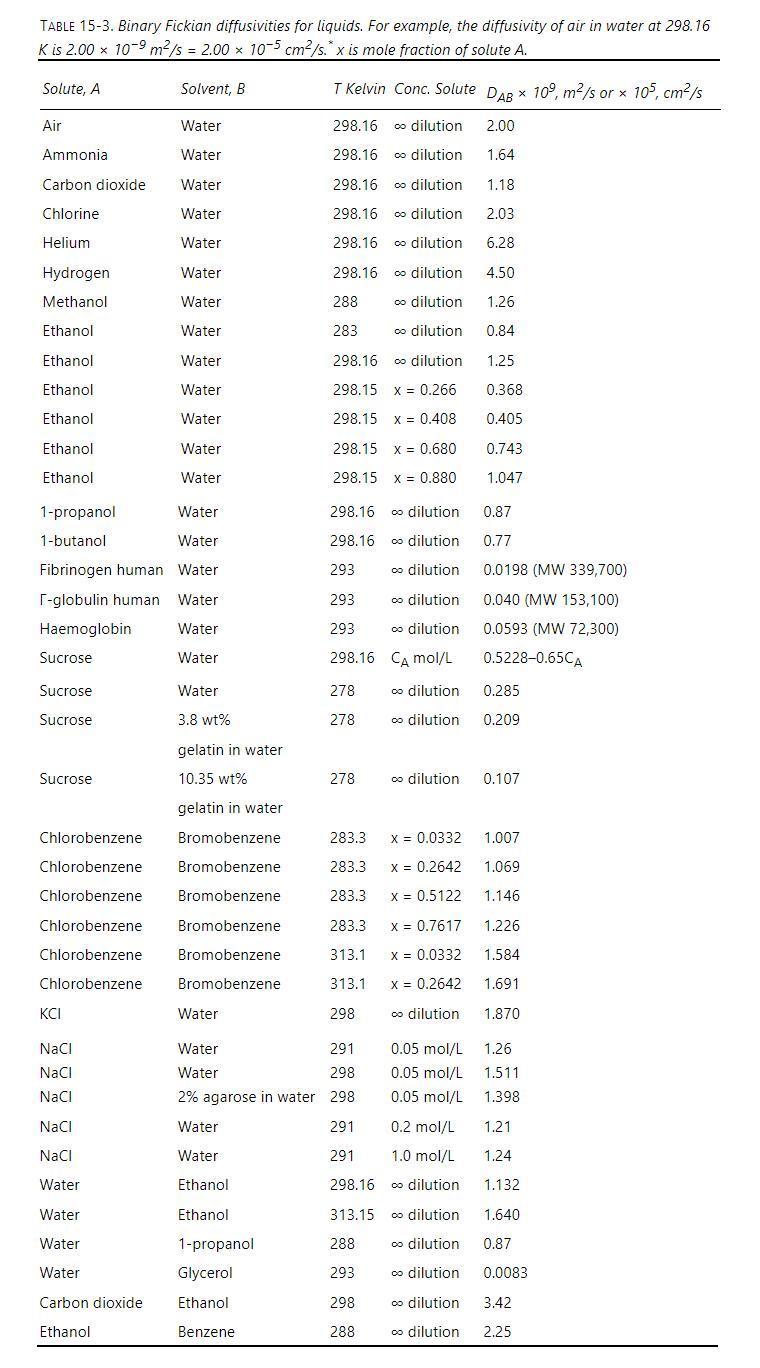
Equation 15-22c
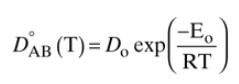
TABLE 15-3. Binary Fickian diffusivities for liquids. For example, the diffusivity of air in water at 298.16 K is 2.00 10-9 m/s = 2.00 10-5 cm/s.* x is mole fraction of solute A. Solute, A Solvent, B T Kelvin Conc. Solute DAB 109, m2/s or 105, cm/s Air Water 298.16 dilution 2.00 Ammonia Water 298.16 co dilution 1.64 Carbon dioxide Water 298.16 co dilution 1.18 Chlorine Water 298.16 dilution 2.03 Helium Water 298.16 co dilution 6.28 Hydrogen Water 298.16 co dilution 4.50 Methanol Water 288 co dilution 1.26 Ethanol Water 283 co dilution 0.84 Ethanol Water 298.16 co dilution 1.25 Ethanol Water 298.15 x = 0.266 0.368 Ethanol Water 298.15 x 0.408 0.405 Ethanol Water 298.15 x 0.680 0.743 Ethanol Water 298.15 x 0.880 1.047 1-propanol Water 298.16 * dilution 0.87 1-butanol Water 298.16 co dilution 0.77 Fibrinogen human Water 293 dilution 0.0198 (MW 339,700) T-globulin human Water 293 dilution 0.040 (MW 153,100) Haemoglobin Water 293 co dilution 0.0593 (MW 72,300) Sucrose Water 298.16 CA mol/L 0.5228-0.65CA Sucrose Water 278 dilution 0.285 Sucrose 3.8 wt% 278 dilution 0.209 gelatin in water Sucrose 10.35 wt% 278 dilution 0.107 gelatin in water Chlorobenzene Bromobenzene 283.3 x = 0.0332 1.007 Chlorobenzene Bromobenzene 283.3 x = 0.2642 1.069 Chlorobenzene Bromobenzene 283.3 x = 0.5122 1.146 Chlorobenzene Bromobenzene 283.3 x = 0.7617 1.226 Chlorobenzene Bromobenzene 313.1 x = 0.0332 1.584 Chlorobenzene Bromobenzene 313.1 x = 0.2642 1.691 KCI Water 298 dilution 1.870 NaCl Water 291 0.05 mol/L 1.26 NaCl Water 298 0.05 mol/L 1.511 NaCl 2% agarose in water 298 0.05 mol/L 1.398 NaCl Water 291 0.2 mol/L 1.21 NaCl Water 291 1.0 mol/L 1.24 Water Ethanol 298.16 co dilution 1.132 Water Ethanol 313.15 co dilution 1.640 Water 1-propanol 288 dilution 0.87 Water Glycerol 293 dilution 0.0083 Carbon dioxide Ethanol 298 dilution 3.42 Ethanol Benzene 288 o dilution 2.25
Step by Step Solution
3.51 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


