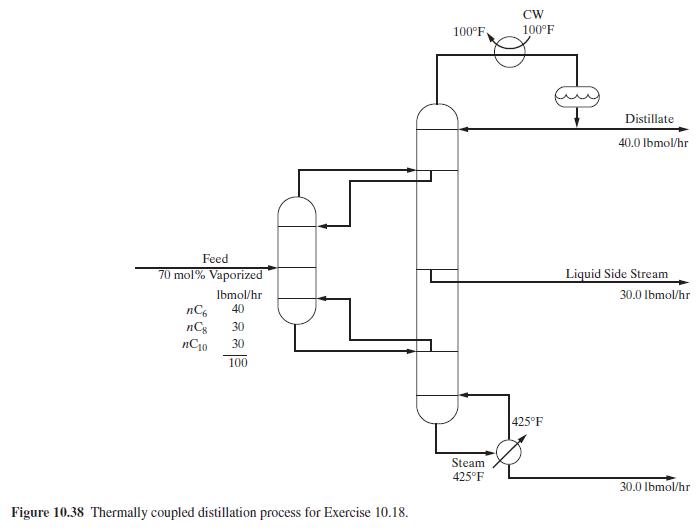
A mixture of three hydrocarbons is to be separated into three nearly pure products by thermally coupled
Question:
A mixture of three hydrocarbons is to be separated into three nearly pure products by thermally coupled distillation at \(1 \mathrm{~atm}\) as shown in Figure 10.38.
Based on the specifications given and other specifications of your choice to achieve reasonably good separations, together with use of the Peng-Robinson equation for thermodynamic properties, use ASPEN PLUS with the MULTIFRAC distillation model to simulate the column. Using the results of the simulation with \(T_{0}=100^{\circ} \mathrm{F}\), calculate the following:
(a) Irreversible production of entropy, \(\mathrm{Btu} / \mathrm{hr}-{ }^{\circ} \mathrm{R}\)
(b) Change in availability function in \(\mathrm{Btu} / \mathrm{hr}\)
(c) Lost work in Btu/hr, kW, and \(\mathrm{Hp}\)
(d) Thermodynamic efficiency
Figure 10.38:-
Step by Step Answer:

Product And Process Design Principles Synthesis Analysis And Evaluation
ISBN: 9781119355243
4th Edition
Authors: Warren D. Seider, Daniel R. Lewin, J. D. Seader, Soemantri Widagdo, Rafiqul Gani, Ka Ming Ng





