Adipic acid is used in the manufacture of nylon 6,6. It is made by hydrogenation of phenol
Question:
Adipic acid is used in the manufacture of nylon 6,6. It is made by hydrogenation of phenol to a mixture of cyclohexanol and cyclohexanone (known as KA oil – ketone and alcohol), followed by oxidation with nitric acid. The overall reaction can be written approximately as:
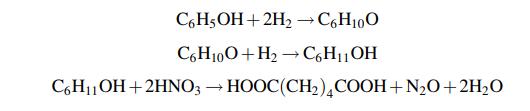
The actual process requirements of phenol, hydrogen, nitric acid and utilities and consumables have been determined to be:
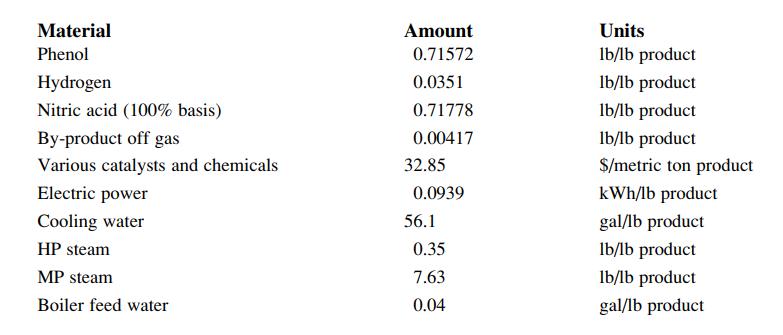
These yields were taken from Chem Systems PERP report 98/99-3 Adipic acid (Chem Systems, 1999). The nitric acid consumption is given on a 100% basis, but 60% nitric acid is used in the process. Estimate the fixed capital cost, the working capital, the cash cost of production and total cost of production for a new 400,000 metric ton per year (400 kt/y) adipic acid plant located in Northeast Asia. The prices of adipic acid, phenol, hydrogen and nitric acid have been forecasted for Northeast Asia as $1400/t, $1000/t, $1100/t and $380/t respectively. Assume a 15% cost of capital and a 10 year project life.
Step by Step Answer:

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler





