As discussed in Example 6.7, toluene (left(mathrm{C}_{7} mathrm{H}_{8} ight)) is to be converted thermally to benzene (left(mathrm{C}_{6}
Question:
As discussed in Example 6.7, toluene \(\left(\mathrm{C}_{7} \mathrm{H}_{8}\right)\) is to be converted thermally to benzene \(\left(\mathrm{C}_{6} \mathrm{H}_{6}\right)\) in a hydrodealkylation reactor. The main reaction is \(\mathrm{C}_{7} \mathrm{H}_{8}+\) \(\mathrm{H}_{2} \rightarrow \mathrm{C}_{6} \mathrm{H}_{6}+\mathrm{CH}_{4}\). An unavoidable side reaction occurs that produces biphenyl: \(2 \mathrm{C}_{6} \mathrm{H}_{6} \rightarrow \mathrm{C}_{12} \mathrm{H}_{10}+\mathrm{H}_{2}\).
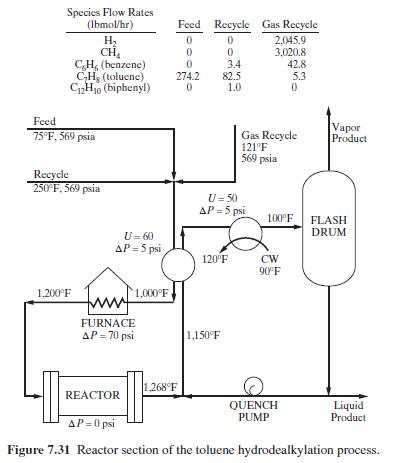
The reactor section of the process is shown in Figure 7.31 as are the conditions for the feed and two recycle streams. The flow rate of the quench stream should be such that the reactor effluent is quenched to \(1,150^{\circ} \mathrm{F}\). Conversion of toluene in the reactor is \(75 \mathrm{~mol} \%\). Two mole percent of the benzene present after the first reaction occurs is converted to biphenyl. Use a process simulator to perform material and energy balances with the Soave-Redlich-Kwong equation of state (RK-SOAVE option in ASPEN PLUS).
Figure 7.31:-
Data From Example 6.7:-
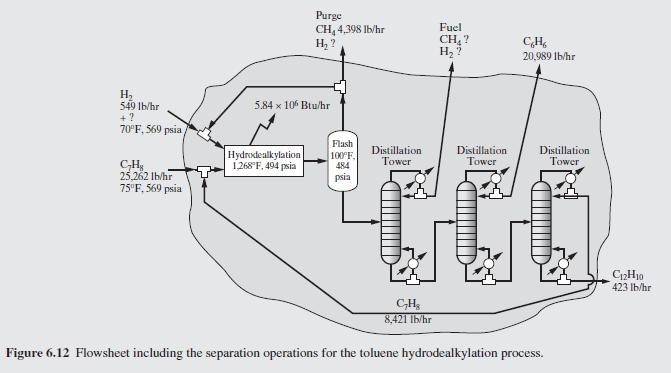
Figure 6.12:-
Step by Step Answer:

Product And Process Design Principles Synthesis Analysis And Evaluation
ISBN: 9781119355243
4th Edition
Authors: Warren D. Seider, Daniel R. Lewin, J. D. Seader, Soemantri Widagdo, Rafiqul Gani, Ka Ming Ng





