The off gases from a gasoline stabilizer are fed to a steam reforming plant to produce hydrogen.
Question:
The off gases from a gasoline stabilizer are fed to a steam reforming plant to produce hydrogen.
The composition of the off gas, molar%, is CH4 77:5, C2H6 9:5, C3H8 8:5, C4H10 4:5.
The gases entering the reformer are at a pressure of 2 bara and 35 8C and the feed rate is 2000 m3/h.
The reactions in the reformer are
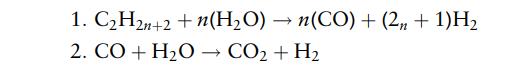
The molar conversion of C2H2n+2 in reaction (1) is 96% and of CO in reaction (2) 92%.
Calculate
i. The average molecular mass of the off gas;
ii. The mass of gas fed to the reformer, kg/h;
iii. The mass of hydrogen produced, kg/h.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





