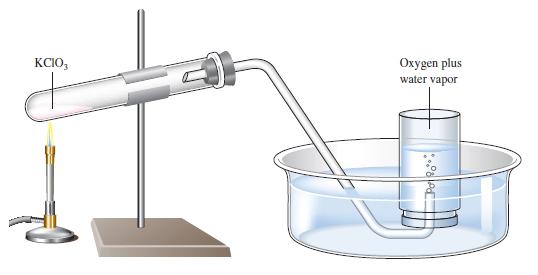
Question: A sample of solid potassium chlorate (KClO 3 ) was heated in a test tube (see Fig. 5.10) and decomposed according to the following reaction:
A sample of solid potassium chlorate (KClO3) was heated in a test tube (see Fig. 5.10) and decomposed according to the following reaction:
![]()
The oxygen produced was collected by displacement of water at 22°C at a total pressure of 754 torr. The volume of the gas collected was 0.650 L, and the vapor pressure of water at 22°C is 21 torr. Calculate the partial pressure of O2 in the gas collected and the mass of KClO3 in the sample that was decomposed.
Figure 5.10
2KCIO3(s) 2KCl(s) + 302(g)
Step by Step Solution
3.55 Rating (155 Votes )
There are 3 Steps involved in it
pressures Thus First we find the partial pressure of O from Daltons law of par... View full answer

Get step-by-step solutions from verified subject matter experts


