Question: temperature change in Metals metal specific heat initial temperature final temperature lead 0.13 aluminum 0.90 Iron 0.45 85C 85C 85C 37C 41C ???????? Three
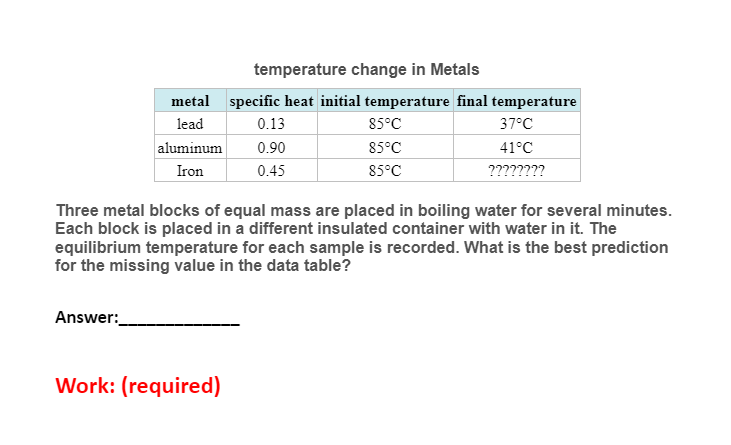
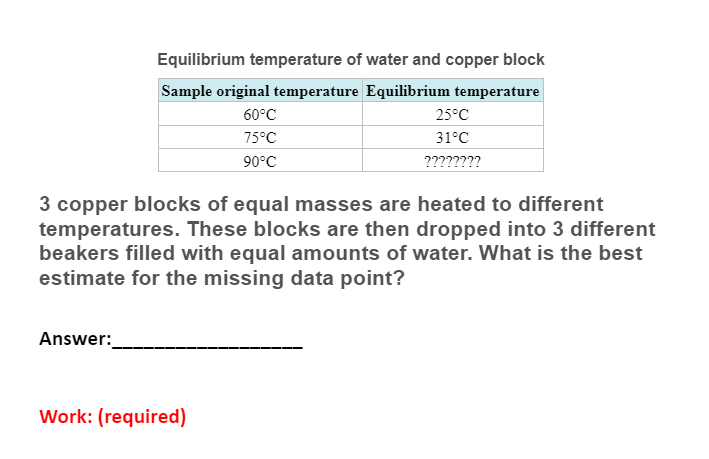
temperature change in Metals metal specific heat initial temperature final temperature lead 0.13 aluminum 0.90 Iron 0.45 85C 85C 85C 37C 41C ???????? Three metal blocks of equal mass are placed in boiling water for several minutes. Each block is placed in a different insulated container with water in it. The equilibrium temperature for each sample is recorded. What is the best prediction for the missing value in the data table? Answer: Work: (required) Equilibrium temperature of water and copper block Sample original temperature Equilibrium temperature 60C 75C 90C 25C 31C ???????? 3 copper blocks of equal masses are heated to different temperatures. These blocks are then dropped into 3 different beakers filled with equal amounts of water. What is the best estimate for the missing data point? Answer: Work: (required)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


