Consider the reaction carried out at 25C and 1 atm. Calculate H S, and G using the
Question:
Consider the reaction
![]()
carried out at 25°C and 1 atm. Calculate ΔH° ΔS°, and ΔG° using the following data:
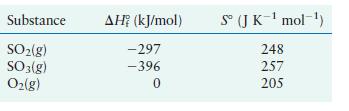
Transcribed Image Text:
2SO2(g) + O2(g) 2SO3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
The value of AH can be calculated from the enthalpies of forma tion usin...View the full answer

Answered By

Akshay Agarwal
I am a Post-Graduate with a specialization in Finance. I have been working in the Consulting industry for the past 8 years with a focus on the Corporate and Investment Banking domain. Additionally, I have been involved in supporting student across the globe in their academic assignments and always strive to provide high quality support in a timely manner. My notable achievements in the academic field includes serving more than 10,000 clients across geographies on various courses including Accountancy, Finance, Management among other subjects. I always strive to serve my clients in the best possible way ensuring high quality and well explained solutions, which ensures high grades for the students along-with ensuring complete understanding of the subject matter for them. Further, I also believe in making myself available to the students for any follow-ups and ensures complete support and cooperation throughout the project cycle. My passion in the academic field coupled with my educational qualification and industry experience has proved to be instrumental in my success and has helped me stand out of the rest. Looking forward to have a fruitful experience and a cordial working relationship.
5.00+
179+ Reviews
294+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Adelyn is in a financial dispute with her creditor. She wants to declare bankruptcy because she is finding herself unable to meet the requirements of paying off her debt. Which court that A would...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
With respect to strategies used by land conservation groups to preserve land, conservation easements seem to be expanding more rapidly than buying land for preservation. In what respect might...
-
Include air resistance for the bales of hay in the previous problem. A bale of hay has a mass of about 30kg and an average area of about 0.2m2. Let the resistance be proportional to the square of the...
-
The pressure in a compressed natural gas line is measured to be 21.6 mmHg. Express this pressure in Pa and psi.
-
In the spring of 1999, Source Associates, Inc. (Source), and Conrad A. Mamajek, Inc. (CAM), entered into a joint venture to act as a middleman for the sale of polymers manufactured by Mitsui...
-
This problem continues the Draper Consulting, Inc., situation from Problem 2-62 of Chapter 2. Start from the trial balance and the posted T-accounts that Draper Consulting, Inc., prepared at December...
-
Explain why not all relationships go through every stage in Knapp's Relational Model. Give an example.
-
Using the following data (at 25C),
-
Calculate S for the reduction of aluminum oxide by hydrogen gas using the following standard entropy values. AlO3(s) + 3H(g) 2Al(s) + 3HO(g)
-
Why do you think the fastest trains are found outside the U.S.? Where is the fastest train operating today?
-
What responsible innovation strategies would we consider if we were creating policies to support innovation in your organization?
-
Portfolio revisions using bond futures contracts. Consider the following original portfolio and revise it with bond futures contracts. Stock 4,000,000 Bonds 4,000,000 Cash 4, 000 000 Total 12,000,000...
-
What are your rights if you are injured at work and your employer does not have workers' compensation? What compensation do you and your dependent children have if your partner dies on a construction...
-
The consultants predict a 25% chance of the project producing a 37% return, a 50% chance of producing a 12% return, and a 25% chance of producing a -18% return. What is the standard deviation given...
-
A single slit of width 0 . 3 mm is illuminated by a mercury light of wavelength 2 5 4 nm . Find the intensity at an 1 1 degree angle to the axis in terms of the intensity of the central maximum.
-
Opech, Inc., produces oil and ships it in a pipeline. On May 1, it had no work-in-process inventory. It started production of 300 million barrels of oil in May and shipped 270 million barrels in the...
-
Suppose that fraction used = / 1.0 + 0.1Mt. for some parameter 1. Write the discrete-time dynamical system and solve for the equilibrium. Sketch a graph of the equilibrium as a function of ....
-
Calculate the vapor pressure of the solvent in each of the following solutions. Use Table 5A.2 to find the vapor pressure of water in (a) An aqueous solution at 100 C in which the mole fraction of...
-
Use Fig. 5B.3 to predict the phase of a sample of CO 2 under the following conditions: (a) 6 atm, 80 C; (b) 1 atm, 56 C; (c) 80. atm, 25 C; (d) 5.1 atm, 56 C. FIGURE 5B.3 73 5.1 Pressure, P/atm 1...
-
Which of the following plots will be linear? (a)[A] against time for a reaction that is first order in A; (b)[A] against time for a reaction that is zeroth order in A; (c)ln [A] against time for a...
-
Which 2 statements are true about Forms 1099 in QuickBooks Online? QuickBooks Online automatically excludes credit card payments from Forms 1099 The same account can be used for more than one box on...
-
Two months ago, a longstanding customer set up an investment portfolio which is recommended for long term investors. He has asked for the investments to be sold even though there is a significant...
-
Booker bond, originally purchased for $533,685. At the end of the first year, the gross carrying amount is $530,609 and a related allowance of $6,000. It is now determined that there has been a...

Study smarter with the SolutionInn App


