Question: Using the following data (at 25C), Cdiamond (s) + O(g) Cgraphite + O(g) - '(s) CO(g) CO(g) AG = -397 kJ AG = -394 kJ
Using the following data (at 25°C),
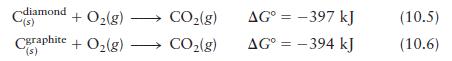
calculate ΔG° for the reaction
![]()
Cdiamond "(s) + O(g) Cgraphite + O(g) - '(s) CO(g) CO(g) AG = -397 kJ AG = -394 kJ (10.5) (10.6)
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
We reverse Equation 106 to make graphite a product as required and then ad... View full answer

Get step-by-step solutions from verified subject matter experts


