For each reaction that is spontaneous under standard conditions (that is, K > 1), write a cell
Question:
For each reaction that is spontaneous under standard conditions (that is, K > 1), write a cell diagram, determine the standard cell potential, and calculate ΔG° for the reaction: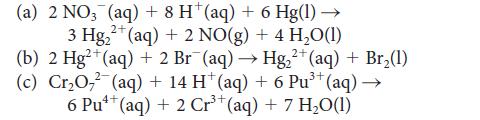
Transcribed Image Text:
(a) 2 NO3 (aq) + 8 H¹ (aq) + 6 Hg(1) → 3 Hg₂2 (aq) + 2 NO(g) + 4 H₂O(1) 2+ 2+ 3+ (b) 2 Hg2+ (aq) + 2 Br¯(aq) → Hg₂²+ (aq) + Br₂(1) (c) Cr₂O7 (aq) + 14 H* (aq) + 6 Pu³+ (aq) → 6 Pu¹(aq) +2 Cr³+(aq) + 7 H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a Ecell 017 V AG 98 kJmol Hg...View the full answer

Answered By

Vijesh J
My passion to become a tutor is a lifetime milestone. Being a finance and marketing professional with hands-on experience in wealth management, portfolio management, team handling and actively contributing in promoting the company. Highly talented in managing and educating students in most attractive ways were students get involved. I will always give perfection to my works. Time is the most important for the works and I provide every answer on time without a delay. I will proofread each and every work and will deliver a with more perfection.
4.70+
5+ Reviews
15+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Solve each formula for the specified variable. (Leave in the answers as needed.) P = kl 00 g for g
-
Ammonia survives indefinitely in air. An agronomist studying how long ammonia survives in soil might need to know whether it survives because its oxidation is not spontaneous under ordinary...
-
(A) Consider a galvanic cell based on the following half-reactions. Assuming the cell operates under standard conditions at 25 C, what is the spontaneous cell reaction? Under what nonstandard...
-
Using the sinking fund Table 13.3, complete the following: Note: Do not round intermediate calculations. Required amount $ Frequency of Length of payment time 8 years 15,000 Annually Interest rate 8...
-
Customer profitability, distribution. Spring Distribution has decided to analyze the profitability of five new customers. It buys bottled water at $12 per case and sells to retail customers at a list...
-
Founded in 1989, Arnold Palmer Hospital is one of the largest hospitals for women and children in the U.S., with 431 beds in two facilities totaling 676,000 square feet. Located in downtown Orlando,...
-
Describe the procedure for examining a hypothesis related to proportions of paired samples.
-
Given the schedule in Table B for a liability work package done as part of an accounting audit in a corporation, find: (a) The critical path. (b) The slack time on process confirmations. (c) The...
-
$ 12,865 The Fiberglass Boat Company reported the following costs and expenses in May 2021: Factory utilities Direct labor $209,860 Depreciation on factory Sales staff salaries 134,520 equipment...
-
Calculate the molar solubility in water of (a) BiI 3 (K sp = 7.71 * 10 19 ); (b) CuCl; (c) CaCO 3 .
-
Write the half-reactions, the balanced equation for the cell reaction, and the cell diagram for each of the following skeletal equations: (a) Ni+(aq) + Zn (s) Ni(s) + Zn+(aq) Ce4+ (b) Ce (aq) + I...
-
Explain the differences in the population growth patterns of the two Paramecium species. What does this tell you about how Paramecium aurelia uses available resources?
-
In this problem, we want to create a spreadsheet to help someone estimate their income in retirement. To do this, we will have them enter several numbers: the amount of money currently in savings,...
-
QUESTION 1 (70 marks) The Lion Group of companies is listed on the JSE. They are a diverse group of companies situated all over Africa. The financial year-end of the Lion Group is 31 December. The...
-
(Binomial model, 23') Consider a European call option with a maturity of 6 months and a strike of $41. The current spot stock price is $40. Consider a two-period binomial model. The stock has annual...
-
1. The force of interest per annum at time t, measured in years from the present, is given by at 1+ at 8(t) = for 0 t8. The accumulated value of 20, 000 invested at time t=0 over 8 years' time is...
-
QUESTION 4 REQUIRED Use the information provided below to prepare the Cash Flow Statement of Nascar Limited for the year ended 31 December 2021. INFORMATION The following Information was extracted...
-
How does standard process costing relate to variance analysis? Is variance analysis likely to be more or less informative in a process-costing setting relative to a job-costing setting?
-
At the beginning of the year, Lam Ltd. had total assets of $800,000 and total liabilities of $500,000. Use this information to answer each of the following independent questions. (a) If Lam's total...
-
Calculate the mean ionic molality, m , in 0.0750 m solutions of a. Ca(NO 3 ) 2 b. NaOH c. MgSO 4 d. AlCl s .
-
A weak acid has a dissociation constant of K a = 2.50 10 2 . a. Calculate the degree of dissociation for a 0.093m solution of this acid using the DebyeHckel limiting law. b. Calculate the degree of...
-
Calculate the mean ionic activity of a 0.0350 m Na 3 PO 4 solution for which the mean activity coefficient is 0.685.
-
4) Brute Force Algorithm Create the Difference cI' Twe Sets Given two arrays of integers A with len[A] = n and Ien[E} = m, create a third array C that includes all elements of A that are not in B. We...
-
'3']: DeereaseandCenquer Algorithm Maximum Element in Array a]: 1iWrite a recursive decreaseandconquer algorithm to calculate the maximum element in a non empty array of real numbers. Your algorithm...
-
6a) Describe the final returned value of the recursive algorithm below on a general input N: int X ( int N) if N

Study smarter with the SolutionInn App


