For the reaction N 2 (g) + 3 H 2 (g) 2 NH 3 (g) at 400.
Question:
For the reaction N2(g) + 3 H2(g) ⇌ 2 NH3(g) at 400. K, K = 41. Find the value of K for each of the following reactions at the same temperature: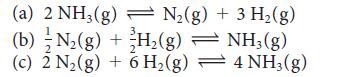
Transcribed Image Text:
(a) 2 NH3(g) N₂(g) + 3 H₂(g) (b) N₂(g) + H₂(g) H₂(g) 2 (c) 2 N₂(g) + 6 H₂(g) NH3(g) 4 NH3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a K 00...View the full answer

Answered By

Thomas Ntim
I am currently, a masters student and tutor at East Tennessee State University where I teach lab sessions, do one on one tutoring session for at least ten students in a week. I have also tutored for the past 6 years at the primary, middle, secondary, and university levels where I used mostly the traditional method of teaching. Thus, standing in front of students to present a clear, organized, and understandable form. Also, my in-depth knowledge of the internet and networking enables me to conduct tutoring online through zoom meetings, emails, and other online platforms. Through my teaching experience, students have soared higher on their educational ladder.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The equilibrium constant for the reaction N2 (g) + O,(g) ;:='02NO(g) is 1.69 x 10-3 at 2300 K. A mixture consisting of 5.0 g of nitrogen and 2.0 g of oxygen in a container of volume 1.0 dm3 is heated...
-
The decomposition of NH3 to N2 and H2 was studied on two surfaces: Without a catalyst, the activation energy is 335 kJ/ mol. a. Which surface is the better heterogeneous catalyst for the...
-
Several factors can impact the structural soundness of 3D-printed objects, including the struts that connect various pieces. The following data appears in the article Analyzing the Effects of...
-
A parallel-plate capacitor has capacitance C0 with no dielectric. It is then filled with dielectric of constant . When a second capacitor of capacitance C' is connected in series with the first one,...
-
1. Using the Buyers Perception of Value presented in Figure 6.1, discuss the value provided by the MDVIP business model. Do you believe that MDVIP offers a good value to patients? 2. Based on the 10...
-
How might a statistician challenge this argument that appeared The New York Times [10] in January 2014? Punishment and surveillance by itself causes people to withdraw from political...
-
Amortization of Intangibles For each of the following intangible assets , indicate the amount of amortization expense that should be recorded for the year 2010 and the amount of accumulated...
-
Find the equation of the line through (-9,-5) which is parallel to the line y = -7x + 6. Give your answer in the form y = mx + b.
-
Hoban worked for Texas Tech as a First Responder Coordinator. She was responsible for training and quality assurance for those responding to nonmedical emergencies, establishing medical protocols for...
-
Nitric oxide, NO, is an intermediate in the production of nitric acid. It is produced commercially by the controlled oxidation of ammonia. Suppose you are considering how to increase the amount of NO...
-
In modern laboratories, sophisticated instruments are used to determine molar mass. However, if you dont have access to such an instrument, you could still determine molar mass using nothing more...
-
Consider the cooling of a glass of tap water by the addition of ice. The glass contains 400 ml of tap water at room temperature, to which 100 gm of ice is added. Assume the glass is adiabatic and...
-
On 1 March 2017, J. Blane Ltd, 7 Down Road, Middlefield, sold the following goods on credit to T. Roy & Son, Ballano Golf Club, Ringlee, Yorkshire: Order No. B/162 4 sets 'Silver Tiger' golf dubs at...
-
You are to enter up the sales, purchases, returns inwards and returns outwards day books from the following details, then to post the items to the relevant accounts in the sales and purchases...
-
A You are to enter the following items in the books, post to personal accounts, and show the transfers to the General Ledger. 2017 1 Credit purchases from: K. Hill 380; M. Norman 500; N. Senior 106....
-
Record the following transactions for the month of January of a small finishing retailer, balance-off all the accounts, and then extract a trial balance as at 31 January 2016. 2016 Jan 1 Started in...
-
Determine the steady-state response for the input \(x(n)=\sin (\omega n) u(n)\) of the filters described by (a) \(y(n)=x(n-2)+x(n-1)+x(n)\) (b) \(y(n)-\frac{1}{2} y(n-1)=x(n)\) (c) \(y(n)=x(n-2)+2...
-
Set up a spreadsheet to solve Problem 1. Determine the estimated cost of the work performed each week given the taskswith their associated costs and schedulesshown in the following table. When a task...
-
What types of questions can be answered by analyzing financial statements?
-
When 2 moles of benzaldehyde are treated with sodium hydroxide, a reaction occurs in which 1 mole of benzaldehyde is oxidized (giving benzoic acid) while the other mole of benzaldehyde is reduced...
-
Predict the major product of each reaction below: (a) (b) (c) 1) EtMgBr 2) H20 :? H. 1) PhMgBr 2) H20
-
Identify the reagents necessary to accomplish each of the transformations below: (a) (b) Me
-
Solve for X: 53x 125 Solve for y 52y+1 23-y What is +y to the nearest hundredth?
-
A container contains 45 green tokens, 5 blue tokens, and 3 red tokens. Two tokens are randomly selected without replacement. Compute P(FIE). E - you select a red token first F-the second token is...
-
How many payments will it take for your bank account to grow to $5000 if you deposit $200 at the end of each month and the account earns 9% compounded monthly?

Study smarter with the SolutionInn App


