In modern laboratories, sophisticated instruments are used to determine molar mass. However, if you dont have access
Question:
In modern laboratories, sophisticated instruments are used to determine molar mass. However, if you don’t have access to such an instrument, you could still determine molar mass using nothing more complicated than a thermometer and a balance. The addition of 0.24 g of sulfur to 100. g of the solvent carbon tetrachloride lowers the solvent’s freezing point by 0.28°C. Sulfur is known to exist in molecular form. What is the molar mass and molecular formula of sulfur molecules?
ANTICIPATE You might already be aware that sulfur commonly forms crownlike S8 molecules.
PLAN Use the procedure for cryoscopy in Toolbox 5F.1. Sulfur is a nonelectrolyte, so i = 1. Once you know the molar mass of the molecules, divide it by the molar mass of sulfur atoms (found in the list of elements inside the back cover) to find how many atoms are in each molecule.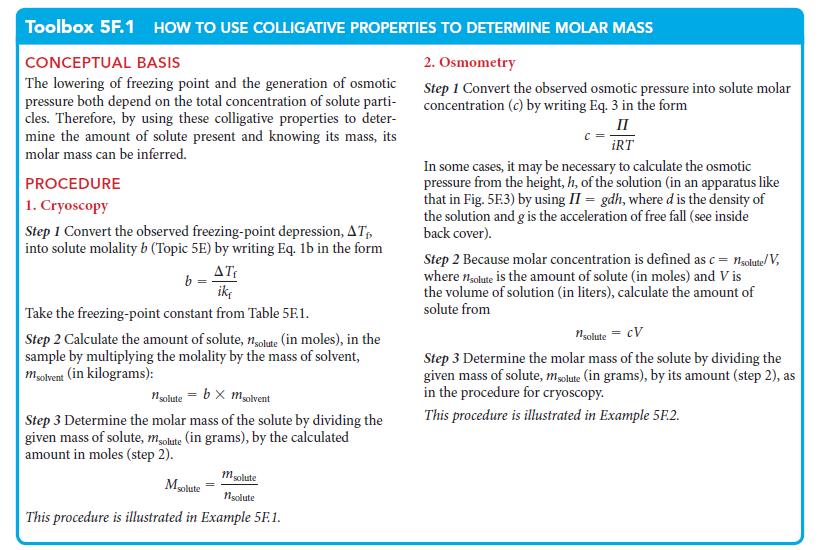
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





