Question: Given the following data: calculate DH for the reaction CH(g) + O(g) C(s) + O(g) H(g) + O(g) - 2CO(g) + HO(l) CO(g) HO(l) AH
Given the following data:
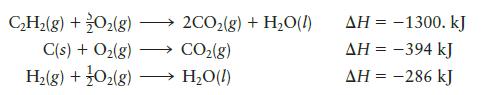
calculate DH for the reaction
![]()
CH(g) + O(g) C(s) + O(g) H(g) + O(g) - 2CO(g) + HO(l) CO(g) HO(l) AH = -1300. kJ AH = -394 kJ AH = -286 kJ
Step by Step Solution
★★★★★
3.50 Rating (153 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Given the following data Eq1 C2H2 g 32O2g CO2g H2O l H... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


