Question: The compound (mathrm{NO}_{2} mathrm{Cl}) is thought to decompose to (mathrm{NO}_{2}) and (mathrm{Cl}_{2}) by the following mechanism: Derive the rate law for the production of (mathrm{Cl}_{2})
The compound \(\mathrm{NO}_{2} \mathrm{Cl}\) is thought to decompose to \(\mathrm{NO}_{2}\) and \(\mathrm{Cl}_{2}\) by the following mechanism:
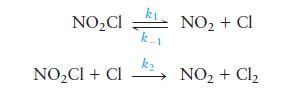
Derive the rate law for the production of \(\mathrm{Cl}_{2}\) using the steady-state approximation.
NOCl NOCI+ CI k_1 NO + Cl NO + Cl
Step by Step Solution
3.48 Rating (148 Votes )
There are 3 Steps involved in it
The steadystate approximation is a useful tool in chemical kinetics for simplifying reaction mechani... View full answer

Get step-by-step solutions from verified subject matter experts


