Question: The reaction between bromate ions and bromide ions in acidic aqueous solution is given by the following equation: Table 15.5 gives the results of four
The reaction between bromate ions and bromide ions in acidic aqueous solution is given by the following equation:
![]()
Table 15.5 gives the results of four experiments involving this reaction. Using these data, determine the orders for all three reactants, the overall reaction order, and the value of the rate constant.
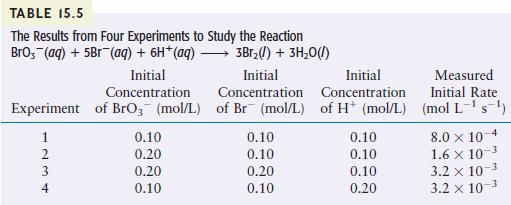
BrO3(aq) + 5Br (aq) + 6H(aq) 3Br2(1) + 3HO(l)
Step by Step Solution
3.40 Rating (147 Votes )
There are 3 Steps involved in it
The general form of the rate law for this reaction is Rate kBrO3 BrH We can determine the values of ... View full answer

Get step-by-step solutions from verified subject matter experts


