The heat capacity of liquid iodine is 80.7 J K21 mol 1 , and its
Question:
The heat capacity of liquid iodine is 80.7 J · K21 · mol–1, and its enthalpy of vaporization is 41.96 kJ · mol–1 at its boiling point (184.3 °C). Using these facts and information in Appendix 2A, calculate the enthalpy of fusion of iodine at 25 °C.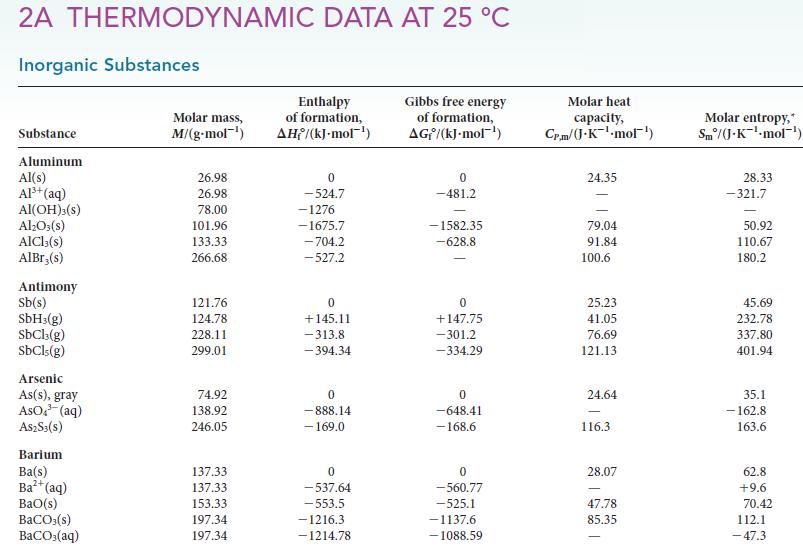
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) AI(OH)3(S) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO³(aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K-¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy,* Sm/(J-K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
12...View the full answer

Answered By

Morgan Njeri
Very Versatile especially in expressing Ideas in writings.
Passionate on my technical knowledge delivery.
Able to multitask and able to perform under pressure by handling multiple challenges that require time sensitive solution.
Writting articles and video editing.
Revise written materials to meet personal standards and satisfy clients demand.
Help Online Students with their course work.
4.90+
12+ Reviews
38+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Suppose you were investigating the correlation between intermolecular forces and the way in which molecules cohere to each other in a liquid and decided that you can gain some insight by comparing...
-
Use data from the Integrative Example to determine how much heat is required to convert 25.00 mL of liquid hydrazine at 25.0 C to hydrazine vapor at its normal boiling point. Integrative Example Use...
-
The heat of vaporization of water at the normal boiling point, 373.2 K, is 40.66 kJ/mol. The specific heat capacity of liquid water is 4.184 JK-1g-1 and of gaseous water is 2.02 J K-1g-1. Assume that...
-
One analogy that is used to think about what is happening in an electric circuit is that of a bucket brigade. Bucket brigades were used before 1900 to fight fires. A group of people would each have a...
-
Daly Inc. reported the following data: Net income $225,000 Depreciation expense 25,000 Gain on disposal of equipment 20,500 Decrease in accounts receivable 14,000 Decrease in accounts payable 3,600...
-
Factor the polynomials. 2x 2 - 12x + 18
-
A partner in your firm has just been offered an appointed to the board of a public listed company that is significantly owned by an overseas shareholder. She has asked you to write her some notes...
-
Lamed Corporation recorded the following transactions for the just completed month. a. $80,000 in raw materials were purchased on account. b. $71,000 in raw materials were requisitioned for use in...
-
The standard interface technology for wireless networks is _ _ _ _ _ _ _ _ _ _ . The hardware component necessary for computers to connect to these networks is a _ _ _ _ _ _ _ _ _ _ .
-
(a) Calculate the change in entropy of a block of copper at 25C that absorbs 65 J of energy from a heater. (b) If the block of copper is at 100. C and it absorbs 65 J of energy from the heater, what...
-
Consider the enthalpies of fusion and the melting points of the following elements: Pb, 5.10 kJ mol 1 , 327 C; Hg, 2.29 kJ mol 1 , 239 C; Na, 2.64 kJ mol 1 , 98 C. Given these data, determine...
-
1. a. Why did the Court of Appeals rule for Johnson, the car dealer? b. Why did the Supreme Court reverse the Court of Appeals judgment that the vehicles air conditioner was not covered by a...
-
The first step in crafting a strategy is the SWOT analysis.True of False
-
E-tailing is synonymous with __________. A. B2B B. B2C C. C2C D. C2B
-
Should an organization change strategies when performance declines? Explain.
-
What types of alternatives can be generated from a SW/OT matrix?
-
How do a SWOT analysis and SW/OT matrix help managers in the strategic decision-making process?
-
Firms A and B make up a cartel that monopolizes the market for a scarce natural resource. The firms marginal costs are MCA = 6 + 2QA and MCB = 18 + QB, respectively. The firms seek to maximize the...
-
Give the structural formulas of the alkenes that, on ozonolysis, give: a. (CH3)2C=O and CH2=O b. Only (CH3CH2)2C=O c. CH3CH=O and CH3CH2CH=O d. O=CHCH2CH2CH2CH=O
-
Calculate the impact rate on a 1.00-cm 2 section of a vessel containing oxygen gas at a pressure of 1.00 atm and 27C.
-
A sample of solid potassium chlorate (KClO 3 ) was heated in a test tube (see Fig. 5.10) and decomposed according to the following reaction: The oxygen produced was collected by displacement of water...
-
The mole fraction of nitrogen in air is 0.7808. Calculate the partial pressure of N 2 in air when the atmospheric pressure is 760. torr.
-
A financial Analyst estimates the required rate of return from a common shareholder in a firm is 11%. The company's dividend just paid (Do) is $1.00 per share, and it will grow at a rate of 25% this...
-
Portfolio revisions using bond futures contracts. Consider the following original portfolio and revise it with bond futures contracts. Stock 4,000,000 Bonds 4,000,000 Cash 4, 000 000 Total 12,000,000...
-
With all of this information in mind, conduct some research on a recession of your choice. Address the following questions: Explain a brief history of the chosen recession. Include the time frame it...

Study smarter with the SolutionInn App


