The magnitudes of the standard potentials of two metals M and X were determined to be When
Question:
The magnitudes of the standard potentials of two metals M and X were determined to be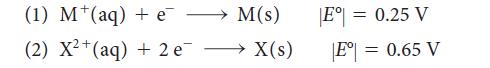
When the two electrodes are connected, current flows from M to X in the external circuit. When the electrode corresponding to half-reaction 1 is connected to the standard hydrogen electrode (SHE), current flows from M to the SHE.
(a) What are the signs of E° of the two half-reactions?
(b) What is the standard cell potential for the cell constructed from these two electrodes?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





