Question: The table below lists the ionic radii for the cations and anions in three different ionic compounds. Each compound has either the NaCl, CsCl, or
The table below lists the ionic radii for the cations and anions in three different ionic compounds.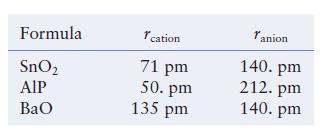 Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of structure formed (NaCl, CsCl, or ZnS) and the type and fraction of holes filled by the cations, and estimate the density of each compound.
Each compound has either the NaCl, CsCl, or ZnS type cubic structure. Predict the type of structure formed (NaCl, CsCl, or ZnS) and the type and fraction of holes filled by the cations, and estimate the density of each compound.
Formula SnO AIP BaO cation 71 pm 50. pm 135 pm Tanion 140. pm 212. pm 140. pm
Step by Step Solution
3.33 Rating (165 Votes )
There are 3 Steps involved in it
the type of structure formed is ZnS Each compound has either the NaCl CsCl or ZnS type cubic structu... View full answer

Get step-by-step solutions from verified subject matter experts


