Use the phase diagram for carbon dioxide (Fig. 5B.3) to predict what would happen to a sample
Question:
Use the phase diagram for carbon dioxide (Fig. 5B.3) to predict what would happen to a sample of carbon dioxide gas at –50°C and 1 atm if its pressure were suddenly increased to 73 atm at constant temperature. What would be the final physical state of the carbon dioxide?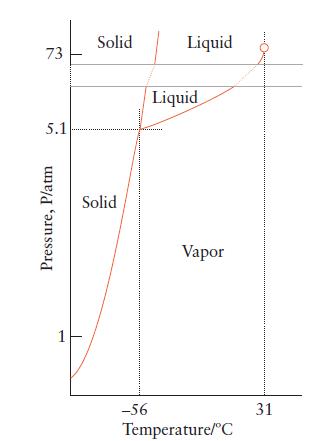
Transcribed Image Text:
73 5.1 Pressure, P/atm 1 Solid Solid -56 Liquid Liquid Vapor Temperature/°C 31
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use the phase diagram for carbon in Exercise 5B.4 (a) To describe the phase transitions that carbon would undergo if the pressure on a sample is increased at a constant temperature of 2000 K from 100...
-
A phase diagram of water is shown at the end of this problem. Label the regions. Predict what would happen as a result of the following changes: (a) Starting at A, we raise the temperature at...
-
Specify the characteristics( r , r and ) of a material in which 100MHz uniform plane wave would have a wavelength of 1m, an attenuation of 2Np/m and an intrinsic impedance of 200 ohms.
-
Use the method of Lagrange multipliers to find these constrained extrema: a. The smallest value of f(x, y) = x 2 + y 2 subject to x + 2y = 4. b. The largest and the smallest values of the function...
-
Subquality natural gas contains an intolerable amount of nitrogen impurity. Separation processes that can be used to remove nitrogen include cryogenic distillation, membrane separation, and...
-
1. What is wrong with this surveillance log? 2. Why is it important to take detailed notes during surveillance and covert operations? The following surveillance log was taken during two fixed-point...
-
Extend the analysis two reactions, making it applicable for steam reforming of methane. The first reaction is \[\mathrm{CH}_{4}+\mathrm{H}_{2} \mathrm{O} ightleftharpoons \mathrm{CO}+3...
-
As an assistant to Jane Melody, Sonics chief marketing officer, youve been assigned to draft a mission statement for top managements review. This should cover the competitive spheres within which the...
-
Labor Supply Define or explain the terms and what they mean to labor supply. Give examples where applicable. A. Utility maximization, preferences, and budget constraint B. Income and substitution...
-
The diagram shows a window made from a rectangle with base 2r m and height h m and a semicircle of radius r m. The perimeter of the window is 6 m and the surface area is A m 2 . a. Express h in terms...
-
The minimum mass concentration of oxygen required for fish life is 4 mg L 1 . (a) Assume the density of lake water to be 1.00 g mL 1 , and express this concentration in parts per million (which is...
-
When 8.05 g of an unknown compound X was dissolved in 100. g of benzene, the vapor pressure of the benzene decreased from 100.0 Torr to 94.8 Torr at 26C. What is (a) The mole fraction and what is (b)...
-
If investors buy properties based on expected future benefits, what is the rationale for appraising a property without making any income or resale price projections?
-
a. Describe the meaning of the term thermodynamic equilibrium. Explain how entropy can be used as a measure of equilibrium and also how other properties can be developed which can be used to assess...
-
Find the probability of selecting someone who does not use drugs. Is the result close to the probability of 0.866 for a negative test result? Refer to the sample data in Table 4-1, which is included...
-
In a recent year, 304 of the approximately 300,000,000 people in the United States were struck by lightning. Estimate the probability that a randomly selected person in the United States will be...
-
It was the start of the new year and Chloe had taken a leap of faith, leaving her full- time job to complete a master's degree. Now, with her research project, she was addressing her long-standing...
-
A: When a day of the week is randomly selected, it is a Saturday. B: When a second different day of the week is randomly selected, it is a Monday. Independent and Dependent Events. (a) determine...
-
What uniform series of cash flows is equivalent to a $15,000 cash flow occurring today if the uniform series of cash flows occur at the end of each year for the next five years and the periodic...
-
Sportique Boutique reported the following financial data for 2012 and 2011. Instructions(a) Calculate the current ratio for Sportique Boutique for 2012 and 2011.(b) Suppose that at the end of 2012,...
-
In order to get in shape for mountain climbing, an avid hiker with a mass of 60. kg ascends the stairs in the worlds tallest structure, the 828 m tall Burj Khalifa in Dubai, United Arab Emirates....
-
A refrigerator is operated by a 0.25-hp (1 hp = 746 watts) motor. If the interior is to be maintained at 4.50C and the room temperature is 38C, what is the maximum heat leak (in watts) that can be...
-
Predict the major product obtained upon radical bromination of each of the following compounds: (a) (b) (c)
-
4. Let G be a pseudorandom generator with expansion factor (n) > 2n. In each of the following cases, say whether G' is necessarily a pseudorandom generator and explain why or why not. Here, "||...
-
Write the code for the del () method in the following doubly linked list class public class ObjDList { private Obj Node list; private Obj Node tail; public ObjDList() { list = null; tail = null; }...
-
Implement the definition of the function rotate ToLeft(), member of the DArray class, so that it rotates all the elements of the array object to the left by one position. Example: [6, 2, 5, 3] [2, 5,...

Study smarter with the SolutionInn App


