Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of
Question:
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following reductions (under standard conditions in acidic solution).
a. reduces \(\mathrm{Cu}^{2+}\) to \(\mathrm{Cu}\) but does not reduce \(\mathrm{Cu}^{2+}\) to \(\mathrm{Cu}^{+}\)
b. reduces \(\mathrm{Br}_{2}\) to \(\mathrm{Br}^{-}\)but does not reduce \(\mathrm{I}_{2}\) to \(\mathrm{I}^{-}\)
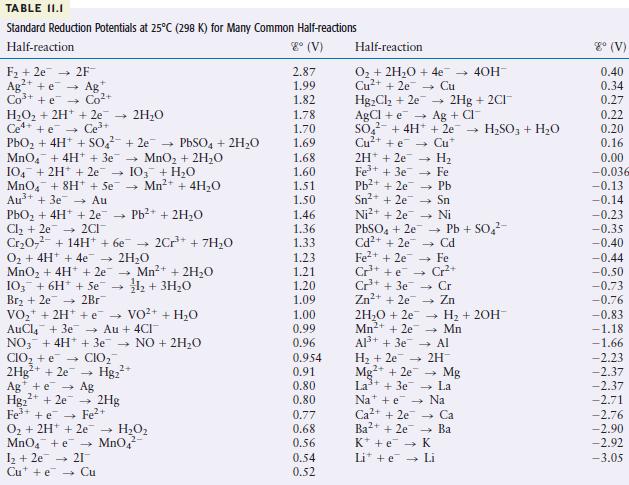
Transcribed Image Text:
TABLE II.I Standard Reduction Potentials at 25C (298 K) for Many Common Half-reactions Half-reaction 8 (V) F +2e 2F Ag+ e Ag Co+ + e Co+ HO + 2H+ + 2e 2HO Ce4+ +e Ce+ PbO + 4H+ + SO +2e PbSO4 + 2HO MnO4 + 4H+ + 3e MnO + 2HO 104 + 2H+ + 2e 103 + HO MnO4 + 8H+ + Se Mn+ + 4HO Au+ + 3e Au PbO + 4H+ + 2e Pb+ + 2HO Cl +2e2CI CrO + 14H+ + 6e 2Cr+ + 7HO O + 4H+ + 4e 2HO MnO + 4H+ + 2e 103 + 6H + Se Br + 2e 2Br VO + 2H+ + VO+ AuCl4 + 3e Au + 4CI NO3 + 4H+ + 3e NO + 2HO CIO + e CIO 2Hg+ + 2e Hg+ - 4 Mn+ + 2HO 1+ 3HO Ag + e Ag 2+ Hg+ + 2e 2Hg Fe+ + e Fe+ O + 2H+ 2e HO MnO4 + e MnO4 1 +2e 21 Cute Cu + HO 2.87 1.99 1.82 1.78 1.70 1.69 1.68 1.60 1.51 1.50 1.46 1.36 1.33 1.23 1.21 1.20 1.09 1.00 0.99 0.96 0.954 0.91 0.80 0.80 0.77 0.68 0.56 0.54 0.52 Half-reaction O + 2HO + 4e Cu+ + 2e Cu HgCl +2e AgCl + e SO4 + 4H+ Cu+ +e 2H+2e7 4 1 Fe+ + 3e7 Pb+ + 2e7 Sn+ + 2e Ni+ + 2e H Fe Pb PbSO4 + 2e7 Cd+ + 2e 2HO +2e Mn+ + 2e Al+ + 3e 2Hg + 2CI+ Ag + CI + 2e HSO3 + HO Cu* - Sn Ni Pb + SO Cd - Fe Cr+ Cr Zn Fe+ + 2e Cr+ + e Cr+ + 3e Zn+ + 2e7 - - 40H- H + 2OH- Mn Al H + 2e 2H Mg2+ + 2e La+ + 3e7 Nae Ca+ + 2e Ba2+ + 2e K + e Li + e Mg La Na Ca Ba K Li 8 (V) 0.40 0.34 0.27 0.22 0.20 0.16 0.00 -0.036 -0.13 -0.14 -0.23 -0.35 -0.40 -0.44 -0.50 -0.73 -0.76 -0.83 -1.18 -1.66 -2.23 -2.37 -2.37 -2.71 -2.76 -2.90 -2.92 -3.05
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To answer these questions we need to look at the table of standard reduction potentials and select reagents that can reduce the specified species with...View the full answer

Answered By

Sufiyan Ahmed Tariq
I am a Chartered Accountant and an Associate Public & Finance Accountant. I also hold a bachelors of Commerce degree. I have over 8 years of experience in accounting, finance and auditing. Through out my career, I have worked with many leading multinational organisation.
I have helped a number of students in studies by teaching them key concepts of subjects like accounting, finance, corporate law and auditing. I help students understanding the complex situation by providing them daily life examples.
I can help you in the following subject / areas:
a) Accounting;
b) Finance;
c) Commerce;
d) Auditing; and
e) Corporate Law.
4.90+
7+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the table of standard reduction potentials (Table 11.1) to pick a reagent that is capable of each of the following oxidations (under standard conditions in acidic solution). a. oxidizes...
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) CIO3(aq) + 5 Cl(aq) + 6 H+ (aq) 3 Cl(g) + 3 HO(l) (b)...
-
Bromine is obtained from brine wells. The process involves treating water containing bromide ion with Cl 2 and extracting the Br 2 from the solution using an organic solvent. Write a balanced...
-
As our energy structure transitions toward renewable fuels, forest-based biomass fuels benefit from this transition. What are the likely effects of this transition on consumers, producers, and the...
-
The motion of a charged particle in an electromagnetic field can be obtained from the Lorentz equation for the force on a particle in such a field, if the electric field vector is E and the magnetic...
-
A storage tank for sulfuric acid is 1.5 m in diameter and 4.0 m high. If the acid has a specific gravity of 1.80, calculate the pressure at the bottom of the tank. The tank is open to the atmosphere...
-
What is the difference between a predator and a situational (accidental) fraudster?
-
FIFO and LIFO Effects You are the vice president of finance of Mickiewicz Corporation, a retail company that prepared two different schedules of gross margin for the first quarter ended March 31,...
-
A 2 kg block is attached to a spring with a force constant of 400 N/m. The block is initially at rest and is compressed by 0.5 meters from its equilibrium position. When released, the block undergoes...
-
The amount of manganese in steel is determined by changing it to permanganate ion. The steel is first dissolved in nitric acid, producing \(\mathrm{Mn}^{2+}\) ions. These ions are then oxidized to...
-
Consider only the species (at standard conditions) \[\mathrm{Na}^{+}, \mathrm{Cl}^{-}, \mathrm{Ag}^{+}, \mathrm{Ag}, \mathrm{Zn}^{2+}, \mathrm{Zn} \text {, and } \mathrm{Pb}\] in answering the...
-
Given the correlation r = 0.307 and the least-squares prediction equation Y = 55.6 + 18.2X, find the predicted value for Y when X is $25.
-
If all state and local pension plans were terminated, with benefits based on past service protected but benefits based on future service replaced by defined contribution pension plans, how many...
-
Very Light Jets (VLJs) are one-pilot, two-engine jets that weigh 10,000 pounds or less and have only five or six passenger seats. Since they cost half as much as the most inexpensive business jets,...
-
Francisco has asked you to provide a memo explaining what risk management techniques you would use to control the property and liability risks you identified in Part I. Your memo should be 3-5 pages...
-
Identify the specific media types that you would utilize to advertise amazon that you identified. Additionally, justify your use of these specific media types and assign a percentage of your media...
-
Blue Mountain Distributors has a $40 million bond outstanding that carries a 12 percent coupon rate paid annually. Current bonds yield are 9.5 percent. The $40 million bond was issued 20 years ago...
-
Tiger Furnishingss CFO believes that a two-stage cost allocation system would give managers better cost information. She asks the companys cost accountant to analyze the accounts and assign overhead...
-
The cost curve for the city water supply is C(Q) = 16 + 1/4 Q2, where Q is the amount of water supplied and C(Q) is the cost of providing Q acre-feet of water. (An acre-foot is the amount of water...
-
Determine Ksp for each of the following sparingly soluble compounds, given their molar solubilities: (a) AgI, 9.1 * 1029 mol L 1 ; (b) Ca(OH) 2 , 0.011 mol L 1 ; (c) Ag 3 PO 4 , 2.7 * 1026 mol L 1...
-
The concentration of mercury, a toxic heavy metal pollutant, in aqueous solution depends in part on the redox properties of its compounds. Suppose you are studying the properties of mercury. You...
-
The following redox reaction is used in acidic solution to prepare orthotelluric acid: Identify the elements undergoing oxidation or reduction and indicate their initial and final oxidation numbers....
-
Problem 1: Recall the simulation in PS5. An axisymmetric rigid body S (spacecraft) moves in an inertial reference frame N. Let , and , be unit vectors fixed in N and S, respectively. Assume that the...
-
Problem 1: Recall the simulation in PS5. An axisymmetric rigid body S (spacecraft) moves in an inertial reference frame N. Let , and , be unit vectors fixed in N and S, respectively. Assume that the...
-
2. The figure below illustrates a mass that is connected to a movable base via a spring and a damper. The positions of the mass and base are defined by x and xb, respectively. Assume that the mass...

Study smarter with the SolutionInn App


