Question: Use the thermodynamic data in Appendix 2A to calculate the acidity constant of HF(aq). 2A THERMODYNAMIC DATA AT 25 C Inorganic Substances Substance Aluminum Al(s)
Use the thermodynamic data in Appendix 2A to calculate the acidity constant of HF(aq).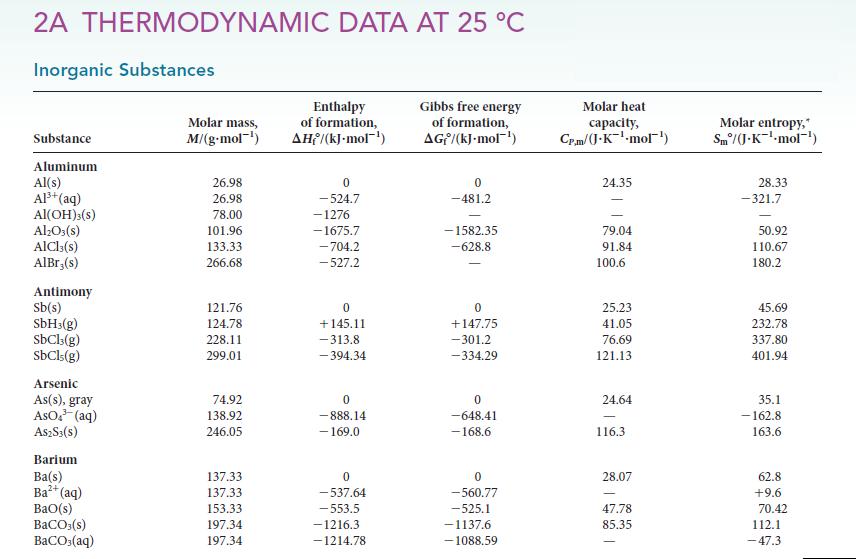
2A THERMODYNAMIC DATA AT 25 C Inorganic Substances Substance Aluminum Al(s) Al+ (aq) AI(OH)3(s) AlO3(s) AICI, (s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls(g) Arsenic As(s), gray AsO4 (aq) A$2S3(S) Barium Ba(s) Ba+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g-mol-) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ.mol-) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ-mol-) 0 -481.2 -1582.35 -628.8 0 +147,75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J-K-mol) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy," Sm/(J.K-mol-) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Step by Step Solution
3.50 Rating (150 Votes )
There are 3 Steps involved in it
K ... View full answer

Get step-by-step solutions from verified subject matter experts


