Use the vapor-pressure curve in Fig. 5A.3 to estimate the boiling point of water when the atmospheric
Question:
Use the vapor-pressure curve in Fig. 5A.3 to estimate the boiling point of water when the atmospheric pressure is
(a) 60. kPa;
(b) 160. kPa.
FIGURE 5A.3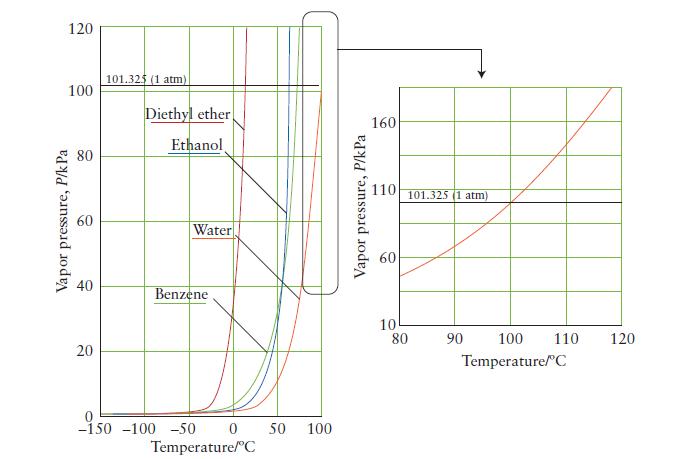
Transcribed Image Text:
120 100 Vapor pressure, P/k Pa 40 20 101.325 (1 atm) Diethyl ether Ethanol Water Benzene -1.50 -150 -100 -50 0 50 100 Temperature/C Vapor pressure, P/kPa 160 110 10 80 101.325 (1 atm) 90 100 110 Temperature/C 120
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use Figure 12.28 to estimate the boiling point of water at an external pressure of 200 torr. (a) 66 C (b) 84 C (c) 100 C (d) 0 C Vapor pressure (torr) 800- 760 600- 400- 200 0- 0 34.6 C Diethyl ether...
-
Refer to Figure 11.5 and determine the boiling point of water at an elevation where the atmospheric pressure is 0.500 atm. Figure 11.5 Vapor pressure (mm Hg) 760 700 600 500 400 300 200 100 0 15...
-
Use data from Table 12.5 to estimate (a) The boiling point of water in Santa Fe, New Mexico, if the prevailing atmospheric pressure is 640 mmHg; (b) The prevailing atmospheric pressure at Lake...
-
Returning to the data set canadaemplmntdata from Problem 17.4, get a line chart of Accommodation jobs by subsetting by VECTOR = v81682. Problem 17.4 The file canadaemplmntdata contains quarterly...
-
(Multiple choice) (1) If the voltage across a parallel-plate capacitor is doubled, its capacitance (a) Doubles. (b) Drops by half. (c) Remains the same. (2) If the charge on an isolated spherical...
-
Contrast the arguments concerning union membership that are likely to be presented by a union with those likely to be presented by an employer.
-
Find the temperature at which the surface tension of water becomes zero. What is the physical significance of this temperature?
-
Massa Company, which has been operating for three years, provides marketing consulting services worldwide for dot-com companies. You are a financial analyst assigned to report on the Massa management...
-
What is the error to this Matlab code for part 3b? Please do parts 3b - 3e Image transcription text WC=1; [-,idx] = min (abs (wout - wc) ) ; A = mag (idx) ; - theta = phaseDeg (idx) ; DO YOUIA WN t...
-
In Exercise 6 of Chapter 2, data on numbers of publications were given for an SRS of 50 faculty members. Not all departments were represented, however, in the SRS. The SRS contained several faculty...
-
Use the information in Table 5G.2 to determine the value of K at 300 K for the reaction 2 BrCl (g) + H 2 (g) Br 2 (g) + 2 HCl(g). TABLE 5G.2 Equilibrium Constants for Various Reactions Reaction H(g)...
-
The following groups are found in some organic molecules. Which are hydrophilic and which are hydrophobic: (a) NH 2 ; (b) CH 3 ; (c) Br; (d) COOH?
-
A worldwide poll on religion was conducted by WIN-Gallup International and published as the document Global Index of Religiosity and Atheism. One question involved religious belief and educational...
-
Compute the \(z\) transform of the following sequences, indicating their regions of convergence: (a) \(x(n)=\sin (\omega n+\theta) u(n)\) (b) \(x(n)=\cos (\omega n) u(n)\) (c) \(x(n)= \begin{cases}n,...
-
Suppose that a company has a portfolio consisting of positions in stocks and bonds. Assume that there are no derivatives. Explain the assumptions underlying (a) the linear model and (b) the...
-
Describe three ways of handling instruments that are dependent on interest rates when the model-building approach is used to calculate VaR. How would you handle these instruments when historical...
-
Identify another research question that Shuang needs to answer and develop some alternative search terms to research this question using a general search engine, a deep web search engine, or an...
-
Encourage students to share examples of their own paperless (or paper-bound) communication styles. How has paper use been encouraged or discouraged in the organizations and companies where they have...
-
Your company completed the East Side subdivision. The costs are shown in Figure 11-4. The site concrete labor and outside lighting were done by subcontractors. The grading and excavation, sanitary...
-
Marc Company assembles products from a group of interconnecting parts. The company produces some of the parts and buys some from outside vendors. The vendor for Part X has just increased its price by...
-
a. Using the relationships derived in Example Problem 7.1 and the values of the critical constants for water from Table 7.2, calculate values for the van der Waals parameters a, b, and R from z c , T...
-
Assign a name for each of the following compounds. Be sure to assign the configuration of each chirality center and indicate the configuration(s) at the beginning of the name. a. b. c. Me
-
Identify the reactants you would use to form a racemic mixture of each of the following epoxides: a. b. c. d. Mery Me
-
A 0.8-specific gravity oil flows through a 32/64-inch choke. Oil viscosity is 1.0 cp. The flowline size is 1.0 inch. The flow coefficient is 1.05. The downstream pressure is 320 psia. Oil flow rate...
-
Calculate the production rate of a well in an oil reservoir at time of 40 days. The following data are given: Flowing bottomhole pressure, p wf =2000 psi Porosity,f= 0.25 Oil saturation S oi = 100%...
-
A 50-ft thick, 30-md permeability sandstone pay zone at a depth of 10000 ft is to be acidized with an acid solution having a specific gravity of 1.07 and a viscosity of 2 cp down a 2-in.-ID coiled...

Study smarter with the SolutionInn App


