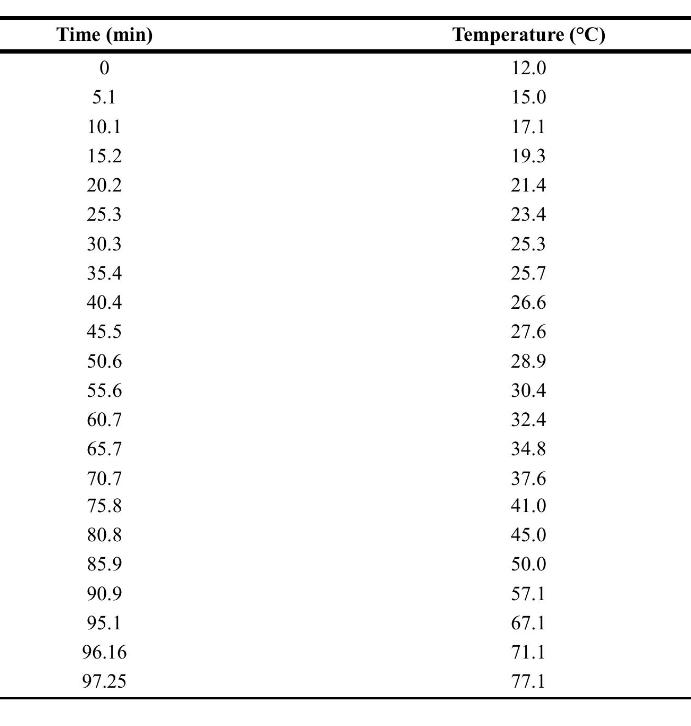
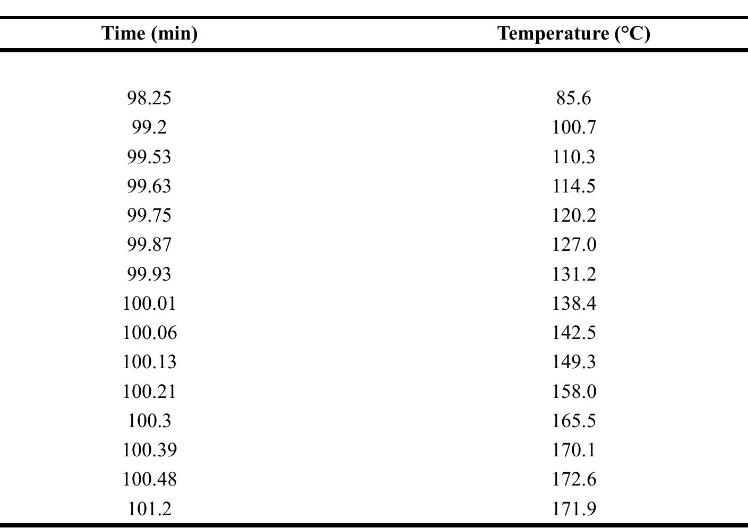
The following data are raw data from the ARSST. Assume a first-order reaction for this system. The
Question:
The following data are raw data from the ARSST. Assume a first-order reaction for this system. The heating rate for the calorimeter is \(0.3^{\circ} \mathrm{C} / \mathrm{min}\).
a. Plot the temperature rate versus \(-1000 / T\) to estimate the detected onset and final temperatures.
b. Use Equation 8-31 to determine the kinetic parameters \(A\) and \(E\) a for the original raw data.
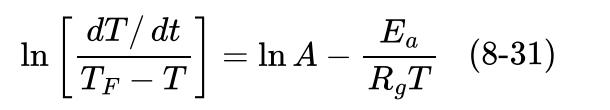
Step by Step Answer:
Related Book For 

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar
Question Posted:




