Hydrogen and O2 can be combined in fuel cells to generate electricity. Solar energy can be used
Question:
Hydrogen and O2 can be combined in fuel cells to generate electricity. Solar energy can be used to split water to generate the raw reactants H2 and O2 for fuel cells. One method of solar thermal reduction is with NiFe2O4 in the sequence Step(1)Solar Energy + NiFe2O4︷Surface(s)→1.2FeO+0.4Fe2O3+NiO︷Solid Solution(s')+0.302↑Step(2)1.2FeO+0.4Fe2O3+NiO︷Solid Solution(s′)+0.6H2O→NiFe2O4︷Surface+0.6H2↑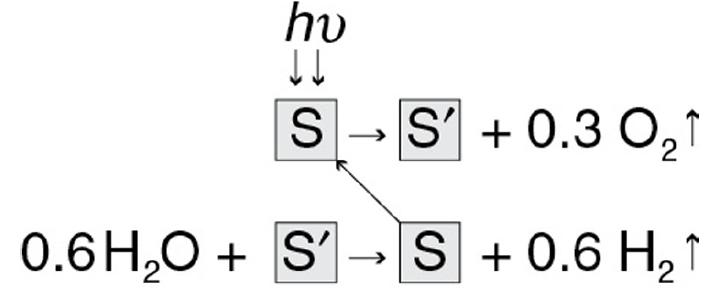
The first equation of the process is as follows: S right arrow S prime plus 0.3 O2 up arrow. Here, S indicates the surface that is NiFe2O4, and S prime indicates the solid solution that is 1.2 Feo plus 0.4 Fe2O3 plus NiO. When Solar energy (h v) falls on the surface, it is converted into a solid solution that reacts with 0.3 moles of Oxygen which is being evolved. The second equation of the process is as follows: 0.6 H2O plus S prime right arrow S plus 0.6 H2 up arrow. Here 0.6 moles of water react with the solid solution which results in the regeneration of the surface (NiFe2O4) and 0.6 moles of Hydrogen have been evolved. We note NiFe2O4 is regenerated in this process. J. R. Scheffe, J. Li, and A. W. Weimer, “A spinel ferrite/hercynite water-splitting redox cycle,” Int. J. Hydrogen Energy, 35, 3333–3340 (2010).
a. Derive a rate law for Step (2), assuming that water adsorbs on the solid solution as a single-site mechanism and that the reaction is irreversible.
b. Repeat (a) when the reaction is reversible and the solid solution adsorption site for water (S′) is different than the NiFe2O4 site for the adsorption of H2, (S). H2O+S′←→S′⋅H2OS′⋅H2O←→S⋅H2+12O2H2⋅S←→S+H2
c. How would your rate law change if we included the effect of hυ in Step 1? S+hυ←→S′⋅O2S′⋅O2←→S′+O2
Step by Step Answer:






