Methyl ethyl ketone (MEK) is an important industrial solvent that can be produced from the dehydrogenation of
Question:
Methyl ethyl ketone (MEK) is an important industrial solvent that can be produced from the dehydrogenation of butan-2-ol (Bu)
over a zinc oxide catalyst (Ind. Eng. Chem. Res., 27, 2050 (1988)):
Bu→catalystMEK+H2 The following data giving the reaction rate for MEK were obtained in a differential reactor at 490°C.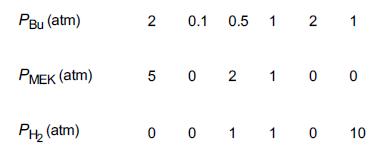
a. Suggest a rate law consistent with the experimental data.
b. Suggest a reaction mechanism and rate-limiting step consistent with the rate law.
c. Plot conversion (up to 90%) and reaction rate as a function of catalyst weight for an entering molar flow rate of pure butan-2-ol of 10 mol/min at an entering pressure P0 = 10 atm up to a catalyst weight Wmax = 23 kg.
d. Repeat part (c), accounting for pressure drop and α = 0.03 kg–1. Plot p and X as a function of catalyst weight down the reactor.
Step by Step Answer:





