Sulfur dioxide is to be removed from a gas by passing the gas and water through a
Question:
Sulfur dioxide is to be removed from a gas by passing the gas and water through a bed of highly porous activated carbon kept at 25°C. In this system sulfur dioxide and oxygen dissolve in water and react on the solid to give sulfur trioxide, as follows: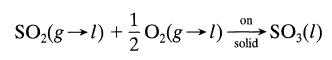
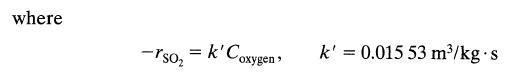
Find the fraction of sulfur dioxide removed from a gas stream under the following conditions: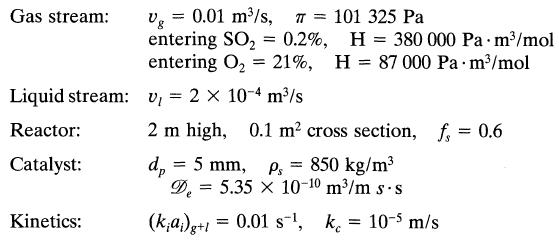
Transcribed Image Text:
(18) ²017 + (18)²0S on solid - SO, (1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To find the fraction of sulfur dioxide removed from the gas stream well first calculate the rate of ...View the full answer

Answered By

Ajeet Singh
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions.
I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life.
I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge.
I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields.
Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a teacher. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students.
4.90+
7+ Reviews
15+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
How important is it for an organization to have a code of conduct that defines fraudulent behavior, and what happens to those individuals who commit such acts? The High Cost of Theft and Fraud Owners...
-
Nitrogen is to be removed from a gas mixture with methane by gas permeation (see Table 1.2) using a glassy polymer membrane that is selective for nitrogen. However, the desired degree of separation...
-
The lowest temperature that has been achieved is about 1 106 K. To achieve this an additional stage of cooling is required beyond that described in the previous problem, namely nuclear cooling. This...
-
Gagnon's Autobody Ltd. repairs and paints automobiles after accidents. Explain how the basic statement of financial position accounts of assets, liabilities, and shareholders' equity would be...
-
The comparative balance sheet of Medalist Athletic Apparel Co. at December 31, 2006 and 2005 is as follows: Additional data obtained from the income statement and from an examination of the accounts...
-
The following transactions occurred in April for Jolly Co.: 201X Apr. 1 Issued check no. 14 for $120 to establish a petty cash fund. 5 Paid $10 from petty cash for postage, voucher no. 1. 8 Paid $19...
-
A PI (Polarization Index) Test of a motor winding is performed and the 10-minute ratio is 3.0. The curve is a perfect parabolic shape. What is the verdict?
-
Can competitors easily replicate Nokias global strategy? Why or why not? What brand of cell phone do you own? If youre living in the United States, chances are it isnt a Nokia. But if youre living...
-
Calculate the levelized cost of heating ($/MBTU) and the emissions (tons/MBTU) for a natural gas furnace and an electric heat pump. [10 points]
-
Consider a different design to effect the hydrogenation of the previous problem, one which uses a long, narrow bubble column of semi suspended 3-mm catalyst particles (f s , = 0.4, f l = 0.5, f g =...
-
The batch hydrogenation takes just about an hour to run. Let us suppose that in practical operations we can run eight batches of fluid per day in this unit. Thus, in the long run a batch of fluid is...
-
Why is open-die forging not a practical technique for large-scale production of identical products?
-
How the CLT can be used to intuitively explain that the error term in multiple regression is normally distributed.
-
On January 1 , Jin owed a debt of $ 3 0 , 2 6 0 . An agreement was reached that she would pay the debt plus compound interest in 2 4 monthly installments of $ 1 , 4 0 0 , the first payment to be made...
-
Why would a person trade the following stocks with references: 1. adidas AG 2. Toyota Industries Corp. ADR 3. Occidental Petroleum Corp. 4. Volvo Car AB Series B 5. CSW Industrials Inc. 6. Bayerische...
-
How does the disease immediately affect the economy and what are some delayed impacts? Only focus on China
-
12 people are in a tennis club. A doubles tennis match consists of two teams of 2 people playing against each other. What is the smallest number of matches that can be played so that everyone gets to...
-
When the price of houses increases, the wage rates for housing construction workers increase. What is the connection?
-
The unadjusted trial balance of Secretarial Services is as follows: SECRETARIAL SERVICES Unadjusted Trial Balance as at 31 December 2017 Account Debit Credit Cash at bank Office supplies Prepaid...
-
The dehydration of n-butyl alcohol (butanol) over an alumina-silica catalyst was investigated by J. F. Maurer (Ph.D. thesis, University of Michigan). The feed consisted of pure butanol. a. Suggest a...
-
The catalytic dehydration of methanol (ME) to form dimethyl ether (DME) and water was carried out over an ion exchange catalyst (K. Klusacek, Collection Czech. Chem. Commun., 49, 170 (1984)). The...
-
In 1981, the U.S. government put forth the following plan for automobile manufacturers to reduce emissions from automobiles over the next few years. All values are in grams per mile. An automobile...
-
There are a number of risk assessment and evaluation strategies that can be applied to dispute management within conveyancing in Australia. Review the strategies below and explain how you can...
-
Bodashka is building a home for Galyna that under the original contract is to be completed by December 31. Galyna found the plans for the home in a publication focusing on unique houses and is...
-
How did the development of agencies, boards, and commissions change the traditional departmental structure of the executive branch of government? . 2. Explain why the development of agencies, boards,...

Study smarter with the SolutionInn App


