Taken from California Professional Engineers Exam. The endothermic liquid-phase elementary reaction A + B 2C proceeds,
Question:
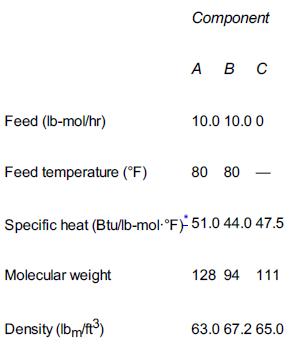
Taken from California Professional Engineer’s Exam. The endothermic liquid-phase elementary reaction A + B → 2C proceeds, substantially, to completion in a single steam-jacketed, continuous-stirred reactor (Table P12-6B). From the following data, calculate the steady-state reactor temperature: Reactor volume: 125 gal Steam jacket area: 10 ft2 Jacket steam: 150 psig (365.9°F saturation temperature) Overall heat-transfer coefficient of jacket, U: 150 Btu/h · ft2 · °F Agitator shaft horsepower: 25 hp Heat of reaction, ΔHRx∘=+20000 Btu/lb-mol of A (independent of temperature)
TABLE P12-6B FEED CONDITIONS AND PROPERTIES
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





