The reversible liquid-phase reaction A B is carried out in a 12-dm 3 CSTR with heat
Question:
The reversible liquid-phase reaction A ⇄ B is carried out in a 12-dm3 CSTR with heat exchange. Both the entering temperature, T0, and the heat exchange fluid, Ta, are at 330 K. An equal molar mixture of inerts and A enter the reactor.
(a) Choose a temperature, T, and carry out a calculation to find G(T) to show that your calculation agrees with the corresponding G(T) value on the curve shown in Figure P12-17B at the temperature you choose.
(b) Find the exit conversion and temperature from the CSTR. X = _____ T = _____ Answers
(c) What entering temperature T0 would give you the maximum conversion? T0 = _____ X = _____
(d) What would the exit conversion and temperature be if the heat-exchange system failed?
(e) Can you find the inlet ignition and extinction temperatures? If yes, what are they? If not, go on to the next problem.
Additional information: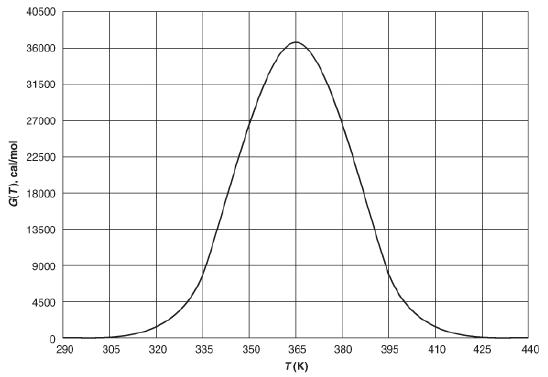
The graph represents the data of heat removed corresponding to the temperature. The horizontal axis represents the temperature in degree Celsius and ranges from 290 to 440 in increments of 15. The vertical axis represents the values of heat removed - G(T) and ranges from 0 to 40500 in increments of 4500. The curve begins at a G(T) value of 0 when the temperature is 290 K. It then goes up and reaches the maximum of 36050 cal/mol when the temperature is 365K. From there, the value drops down to 0 again when the temperature is 440 K. Note that the values are approximate.
Step by Step Answer:






