Determine the wavelength of light emitted when an electron in a hydrogen atom makes a transition from
Question:
Determine the wavelength of light emitted when an electron in a hydrogen atom makes a transition from an orbital in n = 6 to an orbital in n = 5.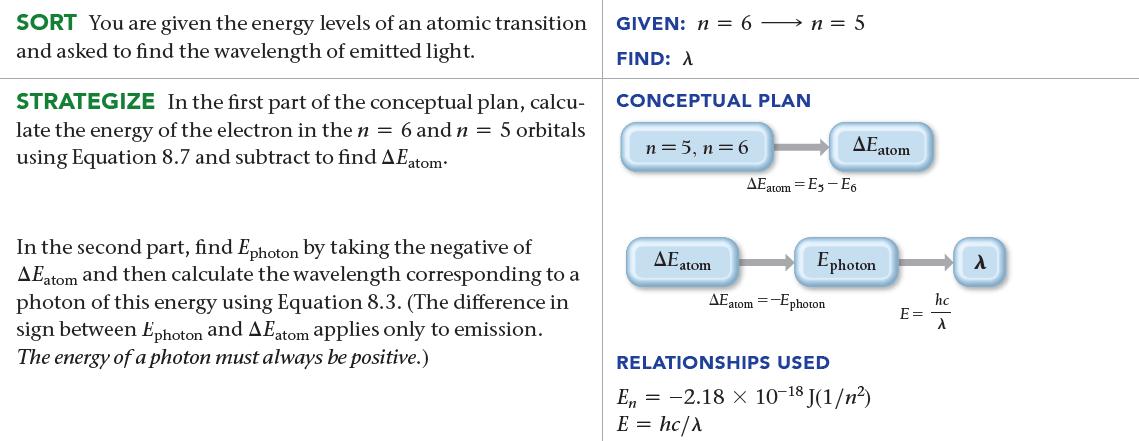
Transcribed Image Text:
SORT You are given the energy levels of an atomic transition and asked to find the wavelength of emitted light. STRATEGIZE In the first part of the conceptual plan, calcu- late the energy of the electron in the n = 6 and n = 5 orbitals using Equation 8.7 and subtract to find AEatom In the second part, find Ephoton by taking the negative of AEatom and then calculate the wavelength corresponding to a photon of this energy using Equation 8.3. (The difference in sign between Ephoton and AEatom applies only to emission. The energy of a photon must always be positive.) GIVEN: n = 6→ n = 5 FIND: A CONCEPTUAL PLAN n = 5, n = 6 AE atom AEatom AE atom-Es-E6 Ephoton AEatom =-Ephoton RELATIONSHIPS USED En = -2.18 × 10-18 J(1/n²) E = hc/λ hc E = - λ λ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
AEatom Es E6 181218 1018 1018 2 218 x 1018 1 5 6 26644 x ...View the full answer

Answered By

RADHIKA MEENAKAR
I am a qualified indian Company Secretary along with Masters in finance with over 6 plus years of professional experience. Apart from this i am a certified accounts and finance tutor on many online platforms.
My Linkedin profile link is here https://www.linkedin.com/in/radhika-meenakar-88b9808a/
5.00+
12+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Determine the wavelength of light emitted when an electron in a hydrogen atom makes a transition from an orbital in n = 5 to n = 4.
-
Calculate the wavelength of the light emitted when an electron in a hydrogen atom makes each transition and indicate the region of the electromagnetic spectrum (infrared, visible, ultraviolet, etc.)...
-
An electron in a hydrogen atom is excited from the ground state to the n = 4 state. Comment on the correctness of the following statements (true or false). (a) n = 4 is the first excited state. (b)...
-
According to a study conducted by the Gallup organization, the proportion of Americans who are afraid to y is 0.10. A random sample of 1100 Americans results in 121 indicating that they are afraid to...
-
Elbert Company was organized on January 1. During the first year of operations, the following plant asset expenditures and receipts were recorded in random order. Debits 1. Cost of real estate...
-
A 6-kg block B starts from rest and slides on the 10-kg wedge A, which is supported by a horizontal surface. Neglecting friction, determine (a) The velocity of B relative to A after it has slid 1 m...
-
Refer to the information in Exercise 17-4. Required 1. Compute a departmental overhead rate for the molding department based on machine hours and a department overhead rate for the trimming...
-
Each of the following items must be considered in preparing a statement of cash flows (indirect method) for Turbulent Indigo Inc. for the year ended December 31, 2014. a) Plant assets that had cost...
-
IKEA entered the United States in 1985 and China in 1998. But the company started in 1958; why did it take so long to move into the United States and China? Why do you think IKEA is not in more than...
-
What determines the color of a colored object? Explain why grass appears green.
-
What values of l are possible for n = 3? (a) 0 (or s) (b) 0 and 1 (or s and p) (c) 0, 1, and 2 (or s, p, and d) (d) 0, 1, 2, and 3 (or s, p, d, and f)
-
Determine if y is a function of x. x + y = 70
-
The risk in bond funds is related to changes in: Multiple choice question. real estate prices interest rates stock market prices
-
An employee who uses their own vehicle for employment purposes cannot deduct any financing costs related to the purchase of the car An employee who is provided with a vehicle owned by the employer...
-
For construction, SME's may also use parametric cost estimates, which are similar to analogous estimates, but utilize scalable cost parameters based on experience in projects in a given industry, for...
-
is it true that " the zero-coupon yield is an equally weight-ed average of forward rates." and the forward rates is an equally weighted average of zero-coupon yield "
-
When the employee accumulates overtime, and you pay it on separation or afterward, make sure to include the insurable earnings in the last pay period of regular pay. Question 19 options: True False
-
The three accounts shown below appear in the general ledger of Herrick Corp. during 2014. Instructions From the postings in the accounts, indicate how the information is reported on a statement of...
-
What is beacon marketing? What are digital wallets?
-
Why can you conclude that the energy of the anti-bonding MO in H + 2 is raised more than the energy of the bonding MO is lowered?
-
The total energy of a molecule is lowered if the orbital energy of the anti-bonding MO is negative, and raised if the orbital energy of the anti-bonding MO is positive. The zero of energy is the...
-
Consider the molecular electrostatic potential map for the LiH molecule shown here. Is the hydrogen atom (shown as a white sphere) an electron acceptor or an electron donor in this molecule?
-
Methodology ( UV sanitizer wand with extension for multi clean ) Use bullets w/paragraphs format to provide details on: - Target Respondents- should be 18 years old minimum up to what age? Your...
-
The Hershey Chocolate Company: Has there been a recent social media outburst based on poor customer experience? What does Google Reviews say about them? Does the company publicly discuss or present...
-
The competition greatly affects the decisions that a company makes regarding how it will position itself in the marketplace. One tool that is commonly used to access the competitive situation and how...

Study smarter with the SolutionInn App


