The graphs labeled (a) and (b) show the titration curves for two equal-volume samples of bases, one
Question:
The graphs labeled (a) and (b) show the titration curves for two equal-volume samples of bases, one weak and one strong. Both titrations were carried out with the same concentration of strong acid.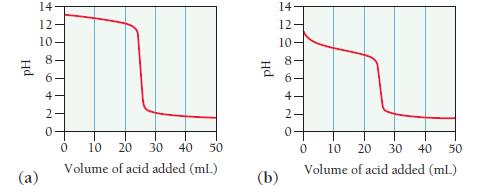
i. What is the approximate pH at the equivalence point of each curve?
ii. Which graph corresponds to the titration of the strong base and which one to the weak base?
Transcribed Image Text:
Hd (a) 14 12- 10. 8 6 4 N 0 10 20 30 40 50 Volume of acid added (mL) 14 12- 10 PH 86 (b) ON 10 20 30 40 50 Volume of acid added (ml) 0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
i Approximate pH at the equivalence point The pH at the equivalence point of a strong basestrong aci...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The graphs labeled (a) and (b) show the titration curves for two equal-volume samples of monoprotic acids, one weak and one strong. Both titrations were carried out with the same concentration of...
-
The graphs below labeled (a) and (b) show the titration curves for two equal-volume samples of monoprotic acids, one weak and one strong. Both titrations were carried out with the same concentration...
-
The accompanying graph shows the titration curves for two monoprotic acids. (a)Which curve is that of a strong acid? (b) What is the approximate pH at the equivalence point of each titration? (c)...
-
If you could choose, which type of school would you want your imaginary child(ren) to attend?
-
In Morgan Company, data concerning two products are: Contribution margin per unitProduct A $11, Product B $12; machine hours required for one unitProduct A 2, Product B 2.5. Compute the Contribution...
-
The following selected transactions were completed during May between Sky Company and Big Co.: May 1. Sky Company sold merchandise on account to Big Co., $72,000, terms FOB destination, 2/15, n/eom....
-
Refer to Samsungs financial statements in Appendix A. Compute its debt ratio as of December 31, 2015, and December 31, 2014. Data From Samsung Financial Statement Appendix A Samsung Electronics Co.,...
-
STOCK ISSUANCE (NONCASH ASSETS, SUBSCRIPTION, AND TREASURY STOCK) Smith & Cline had the following stock transactions during the year: (a) Issued 5,000 shares of common stock with a $5 par value in...
-
Steve consumes two goods, tacos, T, and Red Bull soda, R. His income is $60 per week, the price of tacos is $3 and the price of Red Bull is $2. [2'] A) What is the equation for his budget line?...
-
Two 20.0-mL samples, one 0.200 M KOH and the other 0.200 M CH 3 NH 2 , are titrated with 0.100 M HI. a. What is the volume of added acid at the equivalence point for each titration? b. Is the pH at...
-
Two 25.0-mL samples, one 0.100 M HCl and the other 0.100 M HF, are titrated with 0.200 M KOH. a. What is the volume of added base at the equivalence point for each titration? b. Is the pH at the...
-
Use the spectrochemical series, the oxidation state of the metal, and the geometry of the complex to predict the number of unpaired electrons in [FeCl 4 (H 2 O) 2 ] 2- and [FeCl 4 ] 2- (tetrahedral)....
-
Suppose that people did not believe that the Fed was serious about stopping inflation in 1979, 1980, and 1981. Can you then reconcile the 1981-1982 recession with the Phillips curve and the rational...
-
Explain the known and unknown factors for Risk mitigation. Give examples of the known unknowns and unknown unknowns. List at least five differences between the known and unknown risks involved in the...
-
Could you elucidate the concept of socialization and delineate the intricate process through which individuals undergo personal development within the framework of societal influence and interaction?
-
What trends do you see about businesses and social responsibility? What do you believe are driving these changes? Explain
-
The bond capacity (Face Value) is $100,000 The bond is issued for 5 years Regardless of when the contractor reneges on the successful completion of the job, the draw down on the bind only takes place...
-
Suppose there are 1000 identical wheat farmers. For each, TC = 10 + q2. Market demand is Q = 600,000 - 100p. Derive the short-run equilibrium Q, q, and p. Does the typical firm earn a short- run...
-
For all of the following words, if you move the first letter to the end of the word, and then spell the result backwards, you will get the original word: banana dresser grammar potato revive uneven...
-
Red plane waves from a ruby laser ( 0 = 694.3 nm) in air impinge on two parallel slits in an opaque screen. A fringe pattern forms on a distant wall, and we see the fourth bright band 1.0 above the...
-
Two pinholes in a thin sheet of aluminum are 1.00 mm apart and immersed in a large tank of water (n = 1.33). The holes are illuminated by 0 = 589.3 nm plane waves, and the resulting fringe system is...
-
An expanded beam of red light from a HeNe laser ( 0 = 632.8 nm) is incident on a screen containing two very narrow horizontal slits separated by 0.200 mm. A fringe pattern appears on a white screen...
-
Provide information regarding Russia's foreign exchange regime, foreign exchange rate, and trade account component of the balance of payments. According to McKinsey, currency and exchange rate...
-
Answer this question using diagrams. Consider a version of the labour-leisure problem, where the household's whose preferences satisfy the assumptions given in class. The household's constraints are...
-
It seems that when considering international business pursuits, many US companies make one of two mistakes: 1) they either assume it is much easier than it actually is or 2) they assume it is much...

Study smarter with the SolutionInn App


