The propane fuel (C 3 H 8 ) used in gas barbeques burns according to the thermochemical
Question:
The propane fuel (C3H8) used in gas barbeques burns according to the thermochemical equation: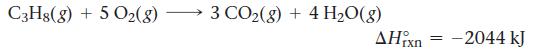
If a pork roast must absorb 1.6 * 103 kJ to fully cook, and if only 10% of the heat produced by the barbeque is actually absorbed by the roast, what mass of CO2 is emitted into the atmosphere during the grilling of the pork roast?
Transcribed Image Text:
C3Hg(g) + 5 O2(g) 3 CO2(g) + 4H2O(g) ΙΧΠ - –2044 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
10...View the full answer

Answered By

Shehar bano
I have collective experience of more than 7 years in education. my area of specialization includes economics, business, marketing and accounting. During my study period I remained engaged with a business school as a visiting faculty member and did a lot of business research. I am also tutoring and mentoring number of international students and professionals online for the last 7 years.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Multinational oil company ExxonMobil faced many challenges related to climate change. Climate change is taking place because of the greenhouse effect. When solar radiation passes through the...
-
In February 19_8, Randy White, president of Arriscraft Corporation (Arriscraft), had just received two requests for a price on two of their marble products. The first request was from a nearby city...
-
Gombas Company decided to analyze certain costs for October of the current year. There was no beginning inventory. Units started into production equaled 14000, units transferred out equal 12000, and...
-
Newell Company has the following internal control procedures over cash disbursements. Identify the internal control principle that is applicable to each procedure. (a) Company checks are prenumbered....
-
What is a wave equation? What is a traveling-wave solution?
-
Judging the Adequacy of Control Procedures Dunbar Camera, Inc., manufactures high-priced motion picture cameras for the movie industry. The component parts specifications are vital to the filmmaking...
-
Helen Weeks has worked for Bonne Consulting Group (BCG) as the executive secretary in the administrative department for nearly 10 years. Her apparent integrity and dedication to her work has quickly...
-
Skydiving terminal velocity. When determining the terminalvelocity of items in free fall, the friction factor f is typicallycalled the drag coefficient and is represented by the symbol CD.They are...
-
In Integrative Case 10.1, we projected financial statements for Walmart Stores, Inc. (Walmart), for Years +1 through +5. In this portion of the Walmart Integrative Case, we use the projected...
-
Charcoal is primarily carbon. Determine the mass of CO 2 produced by burning enough carbon (in the form of charcoal) to produce 5.00 * 10 2 kJ of heat. C(s) + O(8) CO(8) AHxn=-393.5 kJ
-
Titanium reacts with iodine to form titanium(III) iodide, emitting heat. Determine the masses of titanium and iodine that react if 1.55 * 10 3 kJ of heat is emitted by the reaction. 2 Ti(s) + 3 1(g)...
-
What is the real rate of interest of discounting the bill of exchange in Question 27.1? Data From Question 27.1 (a) If you were lent 20,000 for 86 days at 5%, how much interest would you pay? (b) If...
-
2. For each of the following transactions, state two things: (1) which section of the Statement of Cash Flows (SCF) would this transaction be reported in; and (2) would it be ADDED or SUBTRACTED?...
-
Purchase price of the equipment is $460,000. This is a 7 year MACRS asset. Use MACRS method of depreciation. Provide a deprecation schedule for the first four years of the asset. Year Purchase Price...
-
Nora Incorporated sells a single product for $22. Variable costs include $8.58 for each unit plus a 8% sales commission. Fixed costs are $155,290 per month. Required: a. What is the contribution...
-
Coors Company expects sales of $527,000 (6,200 units at $85 per unit). The company's total foxed costs are $264.000 and its versatile costs are $25 per unit. Compute (a) break-even in units and (b)...
-
Last year, Dixon Company produced 11,000 units and sold 9,000 units. The company had no beginning inventory Dixon incurred the following costs: Direct materials per unit Direct labor per unit...
-
Define the three types of responsible trade and explain differences and commonalities.
-
According to a recent survey, 40% of millennials (those born in the 1980s or 1990s) view themselves more as spenders than savers. The survey also reveals that 75% of millennials view social...
-
Radioactive decay can be thought of as an exercise in probability theory. Imagine that you have a collection of radioactive nuclei at some initial time (N 0 ) and are interested in how many nuclei...
-
First-order decay processes as described in the previous problem can also be applied to a variety of atomic and molecular processes. For example, in aqueous solution the decay of singlet molecular...
-
In a subsequent chapter we will encounter the energy distribution P () = Ae /kT , where P () is the probability of a molecule occupying a given energy state, is the energy of the state, k is a...
-
Which cost option has the lowest variable cost? If you only require 25 units of product, which cost option should you choose? Which cost option has the lowest fixed cost? The graph shows 3 different...
-
outline the process (e.g. goal setting, factors to consider, data required, key analysis, stakeholders involved, and judgment logic) that you, as a decision-maker, will use to decide a...
-
Antonia goes to a nature preserve that charges $3 admission, but her mom pays, so it is free for her. She asks permission to buy a pocket knife from the trail shop so she can practice whittling, but...

Study smarter with the SolutionInn App


