Using the vant Hoff factors in Table 14.9, calculate the mass of solute required to make each
Question:
Using the van’t Hoff factors in Table 14.9, calculate the mass of solute required to make each aqueous solution:
a. A sodium chloride solution containing 1.50 * 102 g of water that has a melting point of -1.0 °C
b. 2.50 * 102 mL of a magnesium sulfate solution that has an osmotic pressure of 3.82 atm at 298 K
c. An iron(III) chloride solution containing 2.50 * 102 g of water that has a boiling point of 102 °C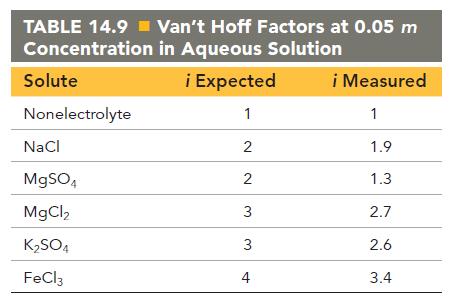
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





